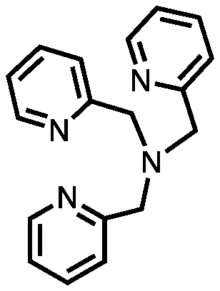
Tris(2-pyridylmethyl)amine
Appearance
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(Pyridin-2-yl)-N,N-bis[(pyridin-2-yl)methyl]methanamine | |
| Other names
TPMA, TPA
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider |
|
| ECHA InfoCard | 100.110.193 |
| MeSH | C431843 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H18N4 | |
| Molar mass | 290.370 g·mol−1 |
| Appearance | Yellowish-pale brown solid |
| Melting point | 73 to 77 °C (163 to 171 °F; 346 to 350 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tris(2-pyridylmethyl)amine (abbreviated TPMA or TPA) is an organic compound with the formula (C5H4NCH2)3N. It is a tertiary amine with three picolyl substituents. It is a white solid that is soluble in polar organic solvents. It is a ligand in coordination chemistry.
The ligand is prepared by the alkylation of 2-picolylamine by picolyl chloride:[1]
- 2 C5H4NCH2Cl + C5H4NCH2NH2 → (C5H4NCH2)3N + 2 HCl
TPMA is a tripodal ligand, often used to simulate the coordination environment within some proteins. It is also used as a copper ligand in ATRP.
Related ligands
[edit]- dipicolylamine, an intermediate in the synthesis of TPMA.
- 2-picolylamine, a bidentate ligand, also known as aminomethylpyridine.
References
[edit]- ^ James W. Canary; Yihan Wang; Richard Roy, Jr. (1998). "Tris[(2-Pyridyl)Methyl] Amine (TPA) and (+)-Bis[(2-Pyridyl)methyl]-1-(2-Pyridyl)-Ethylamine (α-Metpa)". Inorganic Syntheses. Vol. 32. pp. 70–75. doi:10.1002/9780470132630.ch11. ISBN 978-0-470-13263-0.
{{cite book}}:|journal=ignored (help)
