Polysulfobetaine
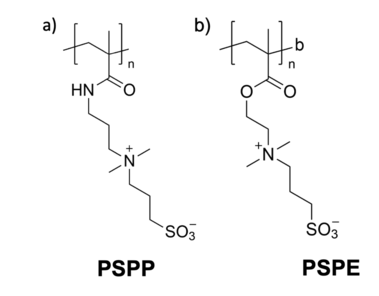
Polysulfobetaines are zwitterionic polymers that contain a positively charged quaternary ammonium and a negatively charged sulfonate group within one constitutional repeat unit.[1][2] In recent years, polysulfobetaines have received increasing attention owing to their good biotolerance and ultralow-fouling behavior towards surfaces. These properties are mainly referred to a tightly bound hydration layer around each zwitterionic group, which effectively suppresses protein adsorption and thus, improves anti-fouling behavior.[3][4] Therefore, polysulfobetaines have been typically employed as ultrafiltration membranes,[4] blood-contacting devices,[5] and drug delivery materials.[3]
The chemical structure of polysulfobetaines can be divided in several subgroups. Most widespread are amides of (meth)acrylic acid ('PSPP') or quaternary esters ('PSPE'). Also, compounds from poly(vinylpyridinium), poly(vinylimidazolium), or quaternary poly(pyrrolidinium) as well as zwitterionic ionenes, are often found.[2][6][7][8][9][10]
Synthesis
[edit]Polysulfobetaines are generally synthesized via free radical polymerization.[6][11] However, the synthesis of polysulfobetaines is often limited by their poor solubility in most solvents and at present, only few sulfobetaine monomers that are suited for free radical polymerization, are commercially available. The most popular ones are SPE and SPP, which provide a good combination of hydrophilicity and polymerizability.[11]
Solution behavior
[edit]Almost all polysulfobetaines are insoluble in water at low temperatures, however many polysulfobetaines feature an upper critical solution temperature (UCST) in aqueous solution. This means they undergo a coil-to-globule collapse transition upon cooling.[12][13] Such a behavior is highly unusual, since other zwitterionic polymers, e.g., poly(phosphatidylcholines) and poly(carboxybetaines) do generally not feature a responsive behavior towards a temperature stimulus.[14][15][16]
The reason for the UCST-type behavior of polysulfobetaines in solution is based on their electrically neutral behavior, i.e., the overall charge is zero, over a large pH range (approximately 2 – 14). Due to the neutralization of the charges, repulsive and attractive interactions are present between the individual polymer chains and inner salt are formed. The balance of this complex interplay of interactions between numerous charged groups with water and with themselves, strongly affects the solubility of polysulfobetaines in water and eventually, results in an UCST-type transition. The temperature of this phase transition, often called clearing point, is very sensitive to molar mass, polymer architecture, solvent isotopes, e.g., H2O/D2O, and especially to the addition of salts to the solution.[17][18][19][20][21][22]
The presence of salt additives in aqueous solution leads to an altered balance of the attractive and repulsive interactions and therefore, also to an altered solubility. Especially, the nature of the salt anion has a strong effect on the solubility of the polysulfobetaines. While chaotropic anions cause an improved dissolution (salting-in effect), kosmotropic anions result in precipitation of the polysulfobetaines (salting-out effect).[23][24][25]
Thin films from polysulfobetaines
[edit]Thin films made from polysulfobetaines also feature a thermo-responsiveness, however, the phase transition is strongly shifted, which is mainly addressed to the increased polymer concentration and the altered polymer-polymer and polymer-water interactions.[12][26][27] Furthermore, and analogously to aqueous solutions, different water isotopes (H2O/D2O) and salt additives were found to affect the phase transition as well.[20][28] Interestingly, polysulfobetaine thin films feature a cononsolvency effect in mixed water/methanol vapors, which is not found in water/methanol solution. Apparently, polysulfobetaines feature a miscibility with lower alcohols at the substance-rich side of their phase diagrams.[29][30]
References
[edit]- ^ Lowe, Andrew B.; McCormick, Charles L. (2002-11-01). "Synthesis and Solution Properties of Zwitterionic Polymers". Chemical Reviews. 102 (11): 4177–4190. doi:10.1021/cr020371t. ISSN 0009-2665. PMID 12428987.
- ^ a b Laschewsky, André (2014-05-23). "Structures and Synthesis of Zwitterionic Polymers". Polymers. 6 (5): 1544–1601. doi:10.3390/polym6051544. ISSN 2073-4360.
- ^ a b Woodfield, Peter A.; Zhu, Yicheng; Pei, Yiwen; Roth, Peter J. (2014-01-28). "Hydrophobically Modified Sulfobetaine Copolymers with Tunable Aqueous UCST through Postpolymerization Modification of Poly(pentafluorophenyl acrylate)". Macromolecules. 47 (2): 750–762. Bibcode:2014MaMol..47..750W. doi:10.1021/ma402391a. hdl:20.500.11937/3990. ISSN 0024-9297.
- ^ a b Wu, Jiang; Lin, Weifeng; Wang, Zhen; Chen, Shengfu; Chang, Yung (2012-05-15). "Investigation of the Hydration of Nonfouling Material Poly(sulfobetaine methacrylate) by Low-Field Nuclear Magnetic Resonance". Langmuir. 28 (19): 7436–7441. doi:10.1021/la300394c. ISSN 0743-7463. PMID 22512533.
- ^ Yuan, Jiang; Huang, Xiaobo; Li, Pengfei; Li, Li; Shen, Jian (2013-08-28). "Surface-initiated RAFT polymerization of sulfobetaine from cellulose membranes to improve hemocompatibility and antibiofouling property". Polymer Chemistry. 4 (19): 5074–5085. doi:10.1039/C3PY00565H. ISSN 1759-9962.
- ^ a b Kudaibergenov, Sarkyt; Jaeger, Werner; Laschewsky, Andre (2006), "Polymeric Betaines: Synthesis, Characterization, and Application", Supramolecular Polymers Polymeric Betains Oligomers, vol. 201, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 157–224, doi:10.1007/12_078, ISBN 978-3-540-31923-8, retrieved 2022-02-22
- ^ Tarannum, Nazia; Singh, Meenakshi (2013-06-01). "Advances in Synthesis and Applications of Sulfo and Carbo Analogues of Polybetaines: A Review". Reviews in Advanced Sciences and Engineering. 2 (2): 90–111. doi:10.1166/rase.2013.1036. ISSN 2157-9121.
- ^ Wielema, T. A.; Engberts, J. B. F. N. (1987). "Zwitterionic polymers—I. Synthesis of a novel series of poly(vinylsulphobetaines). Effect of structure of polymer on solubility in water". European Polymer Journal. 23 (12): 947–950. Bibcode:1987EurPJ..23..947W. doi:10.1016/0014-3057(87)90038-3. ISSN 0014-3057.
- ^ Grassl, B.; Galin, J.C. (1995). "Segmented Poly(tetramethylene oxide) Zwitterionomers and Their Homologous Ionenes. 1. Synthesis, Molecular Characterization, and Thermal Stability". Macromolecules. 28 (21): 7035–7045. Bibcode:1995MaMol..28.7035G. doi:10.1021/ma00125a001.
- ^ Grassl, Bruno; Meurer, Bernard; Scheer, Monique; Galin, Jean Claude (1997-01-01). "Segmented Poly(tetramethylene oxide) Zwitterionomers and Their Homologous Ionenes. 2. Phase Separation through DSC and Solid State 1 H-NMR Spectroscopy". Macromolecules. 30 (2): 236–245. Bibcode:1997MaMol..30..236G. doi:10.1021/ma960643s. ISSN 0024-9297.
- ^ a b Koeberle, P.; Laschewsky, A. (1994-04-01). "Hydrophobically Modified Zwitterionic Polymers: Synthesis, Bulk Properties, and Miscibility with Inorganic Salts". Macromolecules. 27 (8): 2165–2173. Bibcode:1994MaMol..27.2165K. doi:10.1021/ma00086a028. ISSN 0024-9297.
- ^ a b Niebuur, Bart-Jan; Puchmayr, Jonas; Herold, Christian; Kreuzer, Lucas; Hildebrand, Viet; Müller-Buschbaum, Peter; Laschewsky, André; Papadakis, Christine (2018-05-21). "Polysulfobetaines in Aqueous Solution and in Thin Film Geometry". Materials. 11 (5): 850. Bibcode:2018Mate...11..850N. doi:10.3390/ma11050850. ISSN 1996-1944. PMC 5978227. PMID 29883371.
- ^ Hildebrand, Viet; Laschewsky, André; Zehm, Daniel (2014-10-13). "On the hydrophilicity of polyzwitterion poly (N,N-dimethyl-N-(3-(methacrylamido)propyl)ammoniopropane sulfonate) in water, deuterated water, and aqueous salt solutions". Journal of Biomaterials Science, Polymer Edition. 25 (14–15): 1602–1618. doi:10.1080/09205063.2014.939918. ISSN 0920-5063. PMID 25058808. S2CID 23914906.
- ^ Bohrisch, Jörg; Schimmel, Thomas; Engelhardt, Heinz; Jaeger, Werner (2002-05-01). "Charge Interaction of Synthetic Polycarboxybetaines in Bulk and Solution". Macromolecules. 35 (10): 4143–4149. Bibcode:2002MaMol..35.4143B. doi:10.1021/ma0122019. ISSN 0024-9297.
- ^ Bonte, Nelly; Laschewsky, André (1996-05-01). "Zwitterionic polymers with carbobetaine moieties". Polymer. 37 (10): 2011–2019. doi:10.1016/0032-3861(96)87319-8. ISSN 0032-3861.
- ^ Favresse, P; Laschewsky, A (2001-03-01). "Synthesis and investigation of new amphiphilic poly(carbobetaine)s made from diallylammonium monomers". Polymer. 42 (7): 2755–2766. doi:10.1016/S0032-3861(00)00686-8. ISSN 0032-3861.
- ^ Schulz, D. N.; Peiffer, D. G.; Agarwal, P. K.; Larabee, J.; Kaladas, J. J.; Soni, L.; Handwerker, B.; Garner, R. T. (1986-11-01). "Phase behaviour and solution properties of sulphobetaine polymers". Polymer. 27 (11): 1734–1742. doi:10.1016/0032-3861(86)90269-7. ISSN 0032-3861.
- ^ Vishnevetskaya, Natalya S.; Hildebrand, Viet; Niebuur, Bart-Jan; Grillo, Isabelle; Filippov, Sergey K.; Laschewsky, André; Müller-Buschbaum, Peter; Papadakis, Christine M. (2017-05-23). ""Schizophrenic" Micelles from Doubly Thermoresponsive Polysulfobetaine- b -poly( N -isopropylmethacrylamide) Diblock Copolymers". Macromolecules. 50 (10): 3985–3999. Bibcode:2017MaMol..50.3985V. doi:10.1021/acs.macromol.7b00356. ISSN 0024-9297.
- ^ Schmoldt, A.; Benthe, H. F.; Haberland, G. (1975-09-01). "Digitoxin metabolism by rat liver microsomes". Biochemical Pharmacology. 24 (17): 1639–1641. ISSN 1873-2968. PMID 10.
- ^ a b Kreuzer, Lucas P.; Widmann, Tobias; Hohn, Nuri; Wang, Kun; Bießmann, Lorenz; Peis, Leander; Moulin, Jean-Francois; Hildebrand, Viet; Laschewsky, André; Papadakis, Christine M.; Müller-Buschbaum, Peter (2019-05-14). "Swelling and Exchange Behavior of Poly(sulfobetaine)-Based Block Copolymer Thin Films". Macromolecules. 52 (9): 3486–3498. Bibcode:2019MaMol..52.3486K. doi:10.1021/acs.macromol.9b00443. ISSN 0024-9297. S2CID 155174181.
- ^ Hildebrand, Viet; Laschewsky, André; Päch, Michael; Müller-Buschbaum, Peter; Papadakis, Christine M. (2017). "Effect of the zwitterion structure on the thermo-responsive behaviour of poly(sulfobetaine methacrylates)". Polymer Chemistry. 8 (1): 310–322. doi:10.1039/C6PY01220E. ISSN 1759-9954.
- ^ Yu, Jingfeng; Liu, Yudong; Song, Sanan; Gao, Ge; Liu, Fengqi (November 2017). "Phase behavior of a high-concentration sulfobetaine zwitterionic polymer solution". Polymer Journal. 49 (11): 767–774. doi:10.1038/pj.2017.51. ISSN 0032-3896.
- ^ Zhang, Y; Cremer, P (December 2006). "Interactions between macromolecules and ions: the Hofmeister series". Current Opinion in Chemical Biology. 10 (6): 658–663. doi:10.1016/j.cbpa.2006.09.020. PMID 17035073. S2CID 21990688.
- ^ Lo Nostro, Pierandrea; Ninham, Barry W. (2012-04-11). "Hofmeister Phenomena: An Update on Ion Specificity in Biology". Chemical Reviews. 112 (4): 2286–2322. doi:10.1021/cr200271j. ISSN 0009-2665. PMID 22251403.
- ^ Wielema, Thomas A.; Engberts, Jan B. F. N. (1990-01-01). "Zwitterionic polymers—III. Salt effects on the solubility of poly(vinyl sulphobetaines) and poly(vinyl betaines) in aqueous solution". European Polymer Journal. 26 (6): 639–642. Bibcode:1990EurPJ..26..639W. doi:10.1016/0014-3057(90)90220-X. ISSN 0014-3057.
- ^ Kreuzer, Lucas P.; Widmann, Tobias; Aldosari, Nawarah; Bießmann, Lorenz; Mangiapia, Gaetano; Hildebrand, Viet; Laschewsky, André; Papadakis, Christine M.; Müller-Buschbaum, Peter (2020-10-14). "Cyclic Water Storage Behavior of Doubly Thermoresponsive Poly(sulfobetaine)-Based Diblock Copolymer Thin Films". Macromolecules. 53 (20): 9108–9121. Bibcode:2020MaMol..53.9108K. doi:10.1021/acs.macromol.0c01335. ISSN 0024-9297. S2CID 226323489.
- ^ Kreuzer, Lucas P.; Widmann, Tobias; Bießmann, Lorenz; Hohn, Nuri; Pantle, Johannes; Märkl, Raphael; Moulin, Jean-François; Hildebrand, Viet; Laschewsky, André; Papadakis, Christine M.; Müller-Buschbaum, Peter (2020-04-10). "Phase Transition Kinetics of Doubly Thermoresponsive Poly(sulfobetaine)-Based Diblock Copolymer Thin Films". Macromolecules. 53 (8): 2841–2855. Bibcode:2020MaMol..53.2841K. doi:10.1021/acs.macromol.0c00046. ISSN 0024-9297. S2CID 216346530.
- ^ Kreuzer, Lucas P.; Widmann, Tobias; Geiger, Christina; Wang, Peixi; Vagias, Apostolos; Heger, Julian E.; Haese, Martin; Hildebrand, Viet; Laschewsky, André; Papadakis, Christine M.; Müller-Buschbaum, Peter (2021-08-03). "Salt-Dependent Phase Transition Behavior of Doubly Thermoresponsive Poly(sulfobetaine)-Based Diblock Copolymer Thin Films". Langmuir. 37 (30): 9179–9191. doi:10.1021/acs.langmuir.1c01342. ISSN 0743-7463. PMID 34279952. S2CID 236141517.
- ^ Kreuzer, Lucas P.; Geiger, Christina; Widmann, Tobias; Wang, Peixi; Cubitt, Robert; Hildebrand, Viet; Laschewsky, André; Papadakis, Christine M.; Müller-Buschbaum, Peter (2021-07-28). "Solvation Behavior of Poly(sulfobetaine)-Based Diblock Copolymer Thin Films in Mixed Water/Methanol Vapors". Macromolecules. 54 (15): 7147–7159. Bibcode:2021MaMol..54.7147K. doi:10.1021/acs.macromol.1c01179. ISSN 0024-9297. S2CID 237724968.
- ^ Kreuzer, Lucas P.; Lindenmeir, Christoph; Geiger, Christina; Widmann, Tobias; Hildebrand, Viet; Laschewsky, André; Papadakis, Christine M.; Müller-Buschbaum, Peter (2021-01-19). "Poly(sulfobetaine) versus Poly(N-isopropylmethacrylamide): Co-Nonsolvency-Type Behavior of Thin Films in a Water/Methanol Atmosphere". Macromolecules. 54 (3): 1548–1556. Bibcode:2021MaMol..54.1548K. doi:10.1021/acs.macromol.0c02281. ISSN 0024-9297. S2CID 234184714.
