Parisa Mehrkhodavandi
Parisa Mehrkhodavandi | |
|---|---|
| Alma mater | University of British Columbia B.Sc. (1998) Massachusetts Institute of Technology Ph.D. (2002) |
| Scientific career | |
| Institutions | University of British Columbia (2005-present) California Institute of Technology (2002-2005) |
| Thesis | Living α-olefin polymerization by cationic zirconium and hafnium complexes containing chelating diamidopyridine ligands (2002) |
| Doctoral advisor | Richard R. Schrock |
| Other academic advisors | Chris Orvig, John E. Bercaw and Robert H. Grubbs |
Parisa Mehrkhodavandi is a Canadian chemist and Professor of Chemistry at the University of British Columbia (UBC).[1] Her research focuses on the design of new catalysts that can effect polymerization of sustainably sourced or biodegradable polymers.
Education and training
[edit]Parisa Mehrkhodavandi completed her undergraduate degree in chemistry at the University of British Columbia in 1998. During her undergraduate, she worked with Prof. Chris Orvig on the synthesis of novel sugar-containing chelating ligands,[2] and studies on the binding of these ligands to transition metal ions.[3] Mehrkhodavandi also studied cationic lanthanide coordination complexes.[4]
Mehrkhodavandi pursued graduate studies at the Massachusetts Institute of Technology under the supervision of Richard R. Schrock. Her work at MIT focused on the synthesis of cationic zirconium and hafnium complexes bearing arylated diamidopyridine ligands,[5] and the polymerization of 1-hexene with these catalysts.[6][7][8] Mehrkhodavandi graduated with her Ph.D. in 2002.
She conducted a post-doctoral research stint at the California Institute of Technology working together with John E. Bercaw and Robert H. Grubbs. There, she studied the mechanism of a reaction of methanol to triptane with indium(III) iodide and zinc(II) iodide as catalysts.[9][10]
Independent career
[edit]Mehrkhodavandi returned to the University of British Columbia as faculty in 2005 and was later promoted to associate professor in 2013.
Over her career, Mehrkhodavandi has been recognized with numerous awards, including but not limited to:
- Alexander von Humboldt Fellowship (2015)
- UBC Killam Research Fellowship (2015)
- Ichikizaki Travel Award (2008, 2010)
- Government of France Mobility Award (2008)
- NSERC University Faculty Award (2005)
- NSERC Post Graduate Scholarship (2001)
Research
[edit]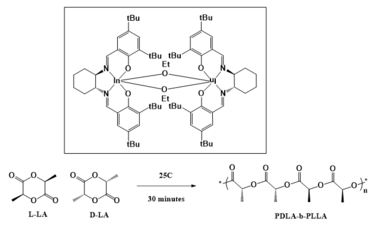
Mehrkhodavandi’s research focuses on catalysis, where her group is pursuing new ligand design strategies. Her work has contributed to new synthetic routes for biodegradable polymers,[11] and fundamental insights into polymerization mechanisms. Her group has a specific interest in the formation of catalysts, such as chiral dinuclear indium complexes, that allow for enantioselective organic reactions.[12][13][14][15] Mehrkhodavandi is also working on the development of biodegradable polyesters using these ligands using cyclic ester monomers. This is being done in three main ways: the first of which is the use of Lewis acid metal centers with chiral ligand supports to open cyclic lactones via ring-opening polymerizations.[16][17] The second is the use of a chiral indium salen catalyst that allows for more precise iso-selectivity similar to chiral aluminum salen catalysts, but with higher activity than aluminum catalysts. The final method utilizes an ethoxy-bridged dinuclear indium catalyst[14] that allows for the creation of diblock copolymers due to its high activity and selective control.
Mehrkhodavandi has patented salen indium catalysts for the ring-opening polymerization of cyclic ester monomers like lactides.[18][19][20]

Publications
[edit]Mehrkhodavandi has published a significant amount of publications over her career. In recent works, Mehrkhodavandi writes about the role of the first alkoxide-bridged indium complex and the zinc analogues as important catalysts in the ring opening polymerization of lactides into polylactic acid.[12] The article pertains to how the indium complex bearing either the chiral or achiral ligand allows for the polymerization of racemic lactide into a highly heterotactic polylactic acid and how the indium complex along with the chiral ligand polymerizes meso-lactide into virtually atactic polylactic acid. Mehrkhodavandi discusses the mechanisms of these reactions in detail, along with the synthesis of the catalysts and activity of the resulting polymers. In another paper, Mehrkhodavandi writes about the use of an indium catalyst as a catalyst for lactide polymerization that has both high activity and high enantioselectivity - other lactide polymerizations feature either high activity or high enantioselectivity.[13] The results demonstrate site control as the primary factor behind the selectivity of the catalyst.
References
[edit]- ^ "Parisa Mehrkhodavandi | UBC Chemistry". www.chem.ubc.ca. Retrieved 2021-06-10.
- ^ Yano, Shigenobu; Shinohara, Yoshie; Mogami, Kaoru; Yokoyama, Mika; Tanase, Tomoaki; Sakakibara, Toru; Nishida, Fumiko; Mochida, Kenichi; Kinoshita, Isamu; Doe, Matsumi; Ichihara, Kanako (2003-05-01). "General Synthesis of Useful Chelating Reagents Having a Sugar Unit, 1,3-Diamino-2-propyl β-D-Glucopyranoside and 1,3-Diamino-2-propyl α-D-Mannopyranoside". Chemistry Letters. 28 (3): 255–256. doi:10.1246/cl.1999.255.
- ^ Song, Bin; Mehrkhodavandi, Parisa; Buglyó, Péter; Mikata, Yuji; Shinohara, Yoshie; Yoneda, Kazumi; Yano, Shigenobu; Orvig, Chris (2000-01-01). "Acid–base and metal ion-binding properties of diaminopropyl D-glucopyranoside and diaminopropyl D-mannopyranoside compounds in aqueous solution". Journal of the Chemical Society, Dalton Transactions (8): 1325–1333. doi:10.1039/A908384G. ISSN 1364-5447.
- ^ Caravan, P.; Mehrkhodavandi, Parisa; Orvig, Chris (1997-03-01). "Cationic Lanthanide Complexes of N,N'-Bis(2-pyridylmethyl)ethylenediamine-N,N'-diacetic Acid (H2bped)". Inorganic Chemistry. 36 (7): 1316–1321. doi:10.1021/ic9613016. ISSN 0020-1669. PMID 11669707.
- ^ Mehrkhodavandi, Parisa; Schrock, Richard R.; Bonitatebus, Peter J. (2002-12-01). "Synthesis and Structures of Zirconium and Hafnium Alkyl Complexes That Contain [H3CC(2-C5H4N)(CH2NAr)2]2- ([ArNpy]2-; Ar = Mesityl, Triisopropylphenyl) Ligands". Organometallics. 21 (26): 5785–5798. doi:10.1021/om0207055. ISSN 0276-7333.
- ^ Mehrkhodavandi, Parisa; Bonitatebus, Peter J.; Schrock, Richard R. (2000-08-01). "A Comparison of Cationic Zirconium Methyl and Isobutyl Initiators that Contain an Arylated Diamido-Pyridine Ligand for Polymerization of 1-Hexene. Elucidation of a Dramatic "Initiator Effect"". Journal of the American Chemical Society. 122 (32): 7841–7842. doi:10.1021/ja000772v. ISSN 0002-7863.
- ^ Mehrkhodavandi, Parisa; Schrock, Richard R. (2001-10-31). "Cationic Hafnium Alkyl Complexes that Are Stable toward β-Hydride Elimination below 10 °C and Active as Initiators for the Living Polymerization of 1-Hexene". Journal of the American Chemical Society. 123 (43): 10746–10747. doi:10.1021/ja0114198. ISSN 0002-7863. PMID 11674011.
- ^ Mehrkhodavandi, Parisa; Schrock, Richard R.; Pryor, Lara L. (2003-10-03). "Living Polymerization of 1-Hexene by Cationic Zirconium and Hafnium Complexes that Contain a Diamido/Donor Ligand of the Type [H3CC(2-C5H4N)(CH2NMesityl)2]2-. A Comparison of Methyl and Isobutyl Initiators". Organometallics. 22 (22): 4569–4583. doi:10.1021/om030438i. ISSN 0276-7333.
- ^ Bercaw, John E.; Diaconescu, Paula L.; Grubbs, Robert H.; Hazari, Nilay; Kay, Richard D.; Labinger, Jay A.; Mehrkhodavandi, Parisa; Morris, George E.; Sunley, Glenn J.; Vagner, Patrick (2007-11-30). "Conversion of Methanol to 2,2,3-Trimethylbutane (Triptane) over Indium(III) Iodide". Inorganic Chemistry. 46 (26): 11371–11380. doi:10.1021/ic7014447. ISSN 0020-1669. PMID 18047325.
- ^ Bercaw, John E.; Diaconescu, Paula L.; Grubbs, Robert H.; Kay, Richard D.; Kitching, Sarah; Labinger, Jay A.; Li, Xingwei; Mehrkhodavandi, Parisa; Morris, George E.; Sunley, Glenn J.; Vagner, Patrick (2006-10-19). "On the Mechanism of the Conversion of Methanol to 2,2,3-Trimethylbutane (Triptane) over Zinc Iodide". The Journal of Organic Chemistry. 71 (23): 8907–8917. doi:10.1021/jo0617823. ISSN 0022-3263. PMID 17081022.
- ^ Xu, Cuiling; Yu, Insun; Mehrkhodavandi, Parisa (2012-06-12). "Highly controlled immortal polymerization of β-butyrolactone by a dinuclear indium catalyst". Chemical Communications. 48 (54): 6806–6808. doi:10.1039/C2CC33114D. ISSN 1364-548X. PMID 22669203.
- ^ a b Kremer, Alexandre B.; Osten, Kimberly M.; Yu, Insun; Ebrahimi, Tannaz; Aluthge, Dinesh C.; Mehrkhodavandi, Parisa (2016-06-06). "Dinucleating Ligand Platforms Supporting Indium and Zinc Catalysts for Cyclic Ester Polymerization". Inorganic Chemistry. 55 (11): 5365–5374. doi:10.1021/acs.inorgchem.6b00358. ISSN 0020-1669. PMID 27187767.
- ^ a b Aluthge, Dinesh C.; Patrick, Brian O.; Mehrkhodavandi, Parisa (2013-04-19). "A highly active and site selective indium catalyst for lactide polymerization". Chemical Communications. 49 (39): 4295–4297. doi:10.1039/C2CC33519K. ISSN 1364-548X. PMID 22729290.
- ^ a b Osten, Kimberly M.; Yu, Insun; Duffy, Ian R.; Lagaditis, Paraskevi O.; Yu, Joey C.-C.; Wallis, Christopher J.; Mehrkhodavandi, Parisa (2012-06-15). "Effects of ligand tuning on dinuclear indium catalysts for lactide polymerization". Dalton Transactions. 41 (26): 8123–8134. doi:10.1039/C2DT30148B. ISSN 1477-9234. PMID 22481250.
- ^ Douglas, Amy F.; Patrick, Brian O.; Mehrkhodavandi, Parisa (2008). "A Highly Active Chiral Indium Catalyst for Living Lactide Polymerization". Angewandte Chemie International Edition. 47 (12): 2290–2293. doi:10.1002/anie.200705033. ISSN 1521-3773. PMID 18273846.
- ^ Broderick, Erin M.; Guo, Neng; Vogel, Carola S.; Xu, Cuiling; Sutter, Jörg; Miller, Jeffrey T.; Meyer, Karsten; Mehrkhodavandi, Parisa; Diaconescu, Paula L. (2011-06-22). "Redox Control of a Ring-Opening Polymerization Catalyst". Journal of the American Chemical Society. 133 (24): 9278–9281. doi:10.1021/ja2036089. ISSN 0002-7863. PMID 21604745.
- ^ Wang, Xinke; Thevenon, Arnaud; Brosmer, Jonathan L.; Yu, Insun; Khan, Saeed I.; Mehrkhodavandi, Parisa; Diaconescu, Paula L. (2014-08-13). "Redox Control of Group 4 Metal Ring-Opening Polymerization Activity toward l-Lactide and ε-Caprolactone". Journal of the American Chemical Society. 136 (32): 11264–11267. doi:10.1021/ja505883u. ISSN 0002-7863. PMID 25062499. S2CID 22098566.
- ^ US 9777023, Mehrkhodavandi, Parisa; Yu, Insun & Acosta-Ramirez, J. Alberto, "Dinuclear indium catalysts and their use for (Co)polymerization of cyclic esters", published 2017-10-03, assigned to University of British Columbia
- ^ US application 20150018493, Mehrkhodavandi, Parisa; Aluthge, Dinesh C. & Clark, Timothy James et al., "Salen indium catalysts and methods of manufacture and use thereof", published 2015-01-15, assigned to Greencentre Canada and University of British Columbia, now abandoned.
- ^ US 10280185, Mehrkhodavandi, Parisa & Aluthge, Dinesh C., "Mononuclear salen indium catalysts and methods of manufacture and use thereof", published 2019-05-07, assigned to University of British Columbia
