PLGA
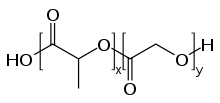
PLGA, PLG, or poly(lactic-co-glycolic) acid (CAS: 26780-50-7 ) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility.[1] PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units (of glycolic or lactic acid) are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.[2]
Copolymer
[edit]Depending on the ratio of lactide to glycolide used for the polymerization, different forms of PLGA can be obtained: these are usually identified in regard to the molar ratio of the monomers used (e.g. PLGA 75:25 identifies a copolymer whose composition is 75% lactic acid and 25% glycolic acid). The crystallinity of PLGAs will vary from fully amorphous to fully crystalline depending on block structure and molar ratio. PLGAs typically show a glass transition temperature in the range of 40-60 °C. PLGA can be dissolved by a wide range of solvents, depending on composition. Higher lactide polymers can be dissolved using chlorinated solvents whereas higher glycolide materials will require the use of fluorinated solvents such as HFIP.
PLGA degrades by hydrolysis of its ester linkages in the presence of water. It has been shown that the time required for degradation of PLGA is related to the monomers' ratio used in production: the higher the content of glycolide units, the lower the time required for degradation as compared to predominantly lactide materials. An exception to this rule is the copolymer with 50:50 monomers' ratio which exhibits the faster degradation (about two months). In addition, polymers that are end-capped with esters (as opposed to the free carboxylic acid) demonstrate longer degradation half-lives.[3] This flexibility in degradation has made it convenient for fabrication of many medical devices, such as, grafts, sutures, implants, prosthetic devices, surgical sealant films, micro and nanoparticles.[4]
PLGA undergoes hydrolysis in the body to produce the original monomers: lactic acid and glycolic acid. These two monomers under normal physiological conditions, are by-products of various metabolic pathways in the body. Lactic acid is metabolized in the tricarboxylic acid cycle and eliminated via carbon dioxide and water. Glycolic acid is metabolized in the same way, and also excreted through the kidney.[5] The body also can metabolize the two monomers, which in the case of glycolic acid produces small amounts of the toxic oxalic acid, though the amounts produced from typical applications are minuscule and there is minimal systemic toxicity associated with using PLGA for biomaterial applications. However, it has been reported that the acidic degradation of PLGA reduces the local pH low enough to create an autocatalytic environment.[6] It has been shown that the pH inside a microsphere can become as acidic as pH 1.5.[7]
Biocompatibility
[edit]Generally PLGA is considered to be quite biocompatible. Its high biocompatibility results from its composition due to lactic and glycolic acid fermentation from sugars, making them eco-friendly and less reactive in the body.[8] PLGA also degrades into non-toxic and non-reactive products that makes them quite useful for various medical and pharmaceutical applications.
The biocompatibility of PLGA has been tested both in vivo and in vitro.[9] The biocompatibility of this polymer is generally determined by the products that it degrades into, as well as the rate of degradation into degradation products. The way that PLGA degrades is by means of an enzyme known as esterase, which forms lactic acid and glycolic acid. These acids then undergo the Krebs Cycle to be degraded as carbon dioxide (CO2) and water (H2O).[10] These byproducts then get removed from the body through cellular respiration and through the digestive process.
While the byproducts usually do not accumulate in the body, there are instances where these byproducts (lactic and glycolic acid) can be dangerous to the body when accumulated in high local concentrations.[11] There can also be small pieces of the polymers as the polymer degrades, causing an immune response by macrophages. These adverse effects can be reduced by using lower concentrations of the polymer, so that it gets naturally released throughout the body.
Something else to consider regarding PLGA biocompatibility is the location at which the polymer is implanted or placed in the body. There are different immune responses that the body could have depending on where the polymer is placed. For example, in drug delivery systems (DDS), PLGA and PLA implants with high surface area and low volume of injection can increase one's chance of immune response as the polymers degrade in the body.
Biodegradability
[edit]The biodegradation of PLGA makes it useful for plenty of medical practices. PLGA undergoes bulk degradation, which is when a catalyst such as water inserts itself throughout the matrix of the polymer.[12] A 75:25 lactide to glycolide PLGA ratio can be made as microspheres that degrade via bulk erosion.[12] This allows degradation throughout the whole polymer to occur equally.
Another injectable form of PLGA was developed to have eroding systems. This form can be used in Lupron Depot. To achieve this, PLGA is mixed with an organic water-miscible solvent approved by the Food and Drug Administration (FDA). Once the PLGA is mixed into the solvent with the drug of choice to create a homogeneous solution or suspension. When this mixture is injected, the PLGA solidifies due to water insolubility and is replaced by the water. Slowly, the drug is delivered from the solution. A problem that may occur is during the initial injection, the drug may be released in a quick burst instead of gradually.[12]
Examples
[edit]Specific examples of PLGA's use include:
- Synthetic Barrier Membrane by Powerbone: This device is a resorbable synthetic membrane that acts as an alternative to polytetrafluoroethylene (PTFE), which is a synthetic polymer often used in dental implants and many other applications.[13] The synthetic barrier membrane is used specifically in dental implants and for guided tissue regeneration (GTR) as well as guided bone regeneration (GBE).[14] Some are biodegradable membranes, while others are not, and are typically correlated with more surgical complications. In general, these membranes are important to provide biocompatibility, biosafety, barrier function, and mechanical properties to the implant. They are also typically bioactive, promoting the regeneration of tissues around the site of implantation.
- Lupron Depot: This is a drug delivery device that helps treat prostate cancer and has been used to treat other types of similar cancers. It is also known as leuprorelin or leuprolide. PLGA is used as a key component in this drug, in the form of microparticles to deliver the drug into the body over a period of 1 week to 6 months.[15] This drug is typically used as an alternative to radiation therapy, and is considered to be quite effective as it reduces the levels of testosterone in the body, slowing the effects of the cancer.[16] There are many side effects of this drug, including muscle loss, hot flashes, fatigue, osteoporosis, growth of breast tissue, and many others.
- Prophylactic delivery: This refers to preventative healthcare that is meant to prevent infections or other illnesses. One case of prophylactic delivery involving PLGA is for the antibiotic vancomycin, which is typically injected after brain surgery to prevent infections from bacteria including Staphylococcus aureus.[17]
See also
[edit]References
[edit]- ^ Abulateefeh SR (February 2023). "Long-acting injectable PLGA/PLA depots for leuprolide acetate: successful translation from bench to clinic". Drug Delivery and Translational Research. 13 (2): 520–530. doi:10.1007/s13346-022-01228-0. PMID 35976565. S2CID 251622670.
- ^ Astete CE, Sabliov CM (2006). "Synthesis and characterization of PLGA nanoparticles". Journal of Biomaterials Science. Polymer Edition. 17 (3): 247–289. doi:10.1163/156856206775997322. PMID 16689015. S2CID 7607080.
- ^ Samadi N, Abbadessa A, Di Stefano A, van Nostrum CF, Vermonden T, Rahimian S, et al. (December 2013). "The effect of lauryl capping group on protein release and degradation of poly(D,L-lactic-co-glycolic acid) particles". Journal of Controlled Release. 172 (2): 436–443. doi:10.1016/j.jconrel.2013.05.034. PMID 23751568.
- ^ Pavot V, Berthet M, Rességuier J, Legaz S, Handké N, Gilbert SC, et al. (December 2014). "Poly(lactic acid) and poly(lactic-co-glycolic acid) particles as versatile carrier platforms for vaccine delivery". Nanomedicine. 9 (17): 2703–2718. doi:10.2217/nnm.14.156. PMID 25529572.
- ^ Crotts G, Park TG (2 July 1998). "Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues". Journal of Microencapsulation. 15 (6): 699–713. doi:10.3109/02652049809008253. PMID 9818948.
- ^ Zolnik BS, Burgess DJ (October 2007). "Effect of acidic pH on PLGA microsphere degradation and release". Journal of Controlled Release. 122 (3): 338–344. doi:10.1016/j.jconrel.2007.05.034. PMID 17644208.
- ^ Fu K, Pack DW, Klibanov AM, Langer R (January 2000). "Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres". Pharmaceutical Research. 17 (1): 100–106. doi:10.1023/A:1007582911958. PMID 10714616. S2CID 22378621.
- ^ Elmowafy EM, Tiboni M, Soliman ME (July 2019). "Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles". Journal of Pharmaceutical Investigation. 49 (4): 347–380. doi:10.1007/s40005-019-00439-x. ISSN 2093-6214. S2CID 256338815.
- ^ Mir M, Ahmed N, Rehman AU (November 2017). "Recent applications of PLGA based nanostructures in drug delivery". Colloids and Surfaces B: Biointerfaces. 159: 217–231. doi:10.1016/j.colsurfb.2017.07.038. PMID 28797972.
- ^ Machatschek R, Lendlein A (March 2020). "Fundamental insights in PLGA degradation from thin film studies". Journal of Controlled Release. 319: 276–284. doi:10.1016/j.jconrel.2019.12.044. PMID 31884098. S2CID 209511941.
- ^ Ramot Y, Haim-Zada M, Domb AJ, Nyska A (December 2016). "Biocompatibility and safety of PLA and its copolymers". Advanced Drug Delivery Reviews. PLA biodegradable polymers. 107: 153–162. doi:10.1016/j.addr.2016.03.012. PMID 27058154.
- ^ a b c Wnek GE, Bowlin GL (2008-05-28). Encyclopedia of Biomaterials and Biomedical Engineering. CRC Press. ISBN 978-1-4987-6143-7.
- ^ "Synthetic Barrier Membrane by Powerbone (Resorbing)". Restore Surgical. Retrieved 2023-04-30.
- ^ Sasaki JI, Abe GL, Li A, Thongthai P, Tsuboi R, Kohno T, Imazato S (May 2021). "Barrier membranes for tissue regeneration in dentistry". Biomaterial Investigations in Dentistry. 8 (1): 54–63. doi:10.1080/26415275.2021.1925556. PMC 8158285. PMID 34104896.
- ^ Park K, Skidmore S, Hadar J, Garner J, Park H, Otte A, et al. (June 2019). "Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation". Journal of Controlled Release. 304: 125–134. doi:10.1016/j.jconrel.2019.05.003. PMID 31071374. S2CID 149444044.
- ^ Fletcher J (6 March 2023). Walton A (ed.). "Lupron (leuprolide acetate) for prostate cancer: What to expect". www.medicalnewstoday.com. Retrieved 2023-04-30.
- ^ Xiao Q, Zhang H, Wu X, Qu J, Qin L, Wang C (2022). "Augmented Renal Clearance in Severe Infections-An Important Consideration in Vancomycin Dosing: A Narrative Review". Frontiers in Pharmacology. 13: 835557. doi:10.3389/fphar.2022.835557. PMC 8979486. PMID 35387348.
External links
[edit] Media related to PLGA at Wikimedia Commons
Media related to PLGA at Wikimedia Commons
