Chlorotoluene
Chlorotoluenes are aryl chlorides based on toluene in which at least one aromatic hydrogen atom is replaced with a chlorine atom. They have the general formula C7H8–nCln, where n = 1–5 is the number of chlorine atoms.
Monochlorotoluene
[edit]Monochlorotoluenes are chlorotoluenes containing one chlorine atom. There are three isomers, each with the formula C7H7Cl.
Properties
[edit]The isomers differ in the location of the chlorine, but have the same chemical formula. All have very similar boiling points, although p-chlorotoluene has a much higher melting point due to a more tightly packed crystal structure.
| Monochlorotoluene isomers | ||||
|---|---|---|---|---|
| Common name | o-chlorotoluene | m-chlorotoluene | p-chlorotoluene | |
| Structure | 
|
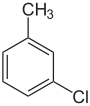
|

| |
| Systematic name | 1-chloro-2-methylbenzene | 1-chloro-3-methylbenzene | 1-chloro-4-methylbenzene | |
| Molecular formula | C7H7Cl (C6H4ClCH3) | |||
| Molar mass | 126.586 g/mol | |||
| Appearance | colorless liquid | |||
| CAS number | [95-49-8] | [108-41-8] | [106-43-4] | |
| Properties | ||||
| Density and phase | 1.073 g/ml, liquid | 1.072 g/ml, liquid | 1.069 g/ml, liquid | |
| Solubility in water | practically insoluble | |||
| Other solubilities | Soluble in non-polar solvents such as aromatic hydrocarbons | |||
| Melting point | −35 °C (−31 °F; 238 K) | −47 °C (−52.6 °F; 226 K) | 7 °C (44.6 °F; 280 K) | |
| Boiling point | 159 °C (318.2 °F; 432 K) | 162 °C (323.6 °F; 435 K) | 162 °C (323.6 °F; 435 K) | |
| Magnetic susceptibility | -81.98 x 10−6 cm3/mol | -80.07 x 10−6 cm3/mol | -80.07 x 10−6 cm3/mol | |
Benzyl chloride is an isomer, which has a chlorine substituted for one of the hydrogens of toluene's methyl group, and it is sometimes named α-chlorotoluene.
Preparation
[edit]A laboratory route to 2- and 4-chlorotoluene proceeds from 2- and 4-toluidines (i.e. 2- and 4-aminotoluene). These compounds are diazotized followed by treatment with cuprous chloride.[1] Industrially, the diazonium method is reserved for 3-chlorotoluene. The industrial route to 2- and 4-chlorotoluene entails direct reaction of toluene with chlorine. The more valuable 4-chlorotoluene is separated from 2-chlorotoluene by distillation. Distillation cannot be applied to separating 3-chlorotoluene from 4-chlorotoluene.[2]
Uses
[edit]2- and 4-chlorotoluene are precursors to the corresponding benzyl chloride (ClC6H4CH2Cl), benzaldehyde (ClC6H4CHO), and benzoyl chloride (ClC6H4C(O)Cl).[2] 2- and 4-chlorotoluenes are converted to 2-chlorobenzonitrile and 4-chlorobenzonitrile, respectively.[3] Chlorotoluenes are precursors to dichlorotoluenes.
See also
[edit]References
[edit]- ^ C. S. Marvel and S. M. McElvain (1923). "o-Chlorotoluene and p-Chlorotoluene". Organic Syntheses. 3: 33. doi:10.15227/orgsyn.003.0033.
- ^ a b Beck, Uwe; Löser, Eckhard (2011). "Chlorinated Benzenes and Other Nucleus-Chlorinated Aromatic Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o03. ISBN 978-3527306732.
- ^ Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363. ISBN 3527306730.
