Talk:Atomic nucleus
Appearance
(Redirected from Talk:Nuclear model)
| This It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
| This is the talk page for discussing improvements to the Atomic nucleus article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| Archives: 1 |
20% change from a constant
[edit]article states:
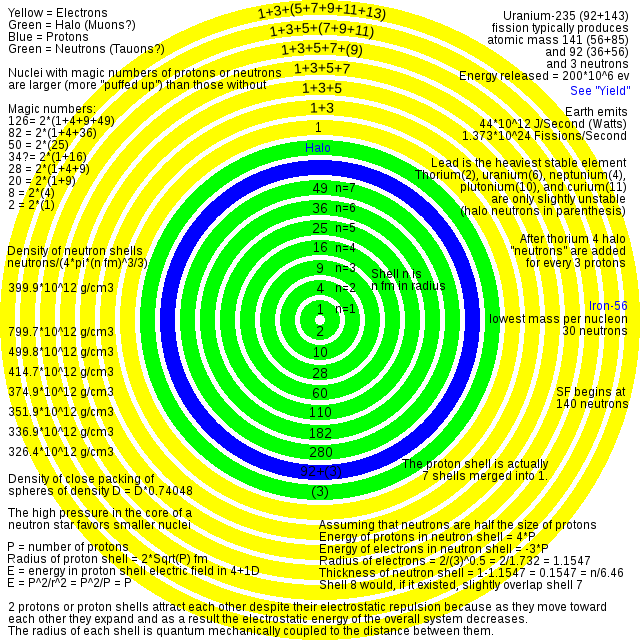
The stable nucleus has approximately a constant density and therefore the nuclear radius R can be approximated by the following formula, where A = Atomic mass number (the number of protons Z, plus the number of neutrons N) and r0 = 1.25 fm = 1.25 × 10−15 m. In this equation, the constant r0 varies by 0.2 fm, depending on the nucleus in question, but this is less than 20% change from a constant.
I believe I can explain why the fudge factor varies by 20% (from 1 to 1.35)
https://upload.wikimedia.org/wikipedia/commons/d/d1/Uranium_atom.svg
Just granpa (talk) 02:21, 18 January 2017 (UTC)
- That sounds like WP:original research, is this publiched clearly somehwhere on a reliable source? Graeme Bartlett (talk) 01:56, 20 July 2018 (UTC)
The nucleus shown in the picture
[edit]I'm pretty sure it's oxygen :) 129.222.29.177 (talk) 22:28, 26 August 2024 (UTC)
- Nine visible blue balls, and six visible red balls. Not so obvious that it even makes a sphere. But the main thing about diagrams like this, is that they make it look like protons and neutrons are just sitting there. As with electrons, they move at high speed, up to the Fermi velocity. Also, they don't show symmetry. He4 has a spherically symmetric nucleus. I believe also O16. That is, when the next state with full shells occurs. A fuzzy purple ball would be a better model. Gah4 (talk) 22:55, 28 August 2024 (UTC)
Categories:
- B-Class vital articles
- Wikipedia level-4 vital articles
- Wikipedia vital articles in Physical sciences
- B-Class level-4 vital articles
- Wikipedia level-4 vital articles in Physical sciences
- B-Class vital articles in Physical sciences
- B-Class physics articles
- Top-importance physics articles
- B-Class physics articles of Top-importance