Hexafluorosilicic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexafluorosilicic acid | |
| Systematic IUPAC name
Dihydrogen hexafluorosilicate | |
| Other names
Fluorosilicic acid, fluosilic acid, hydrofluorosilicic acid, silicofluoride, silicofluoric acid, oxonium hexafluorosilanediuide, oxonium hexafluoridosilicate(2−)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.289 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1778 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| F6H2Si | |
| Molar mass | 144.091 g·mol−1 |
| Appearance | transparent, colorless, fuming liquid |
| Odor | sour, pungent |
| Density | 1.22 g/cm3 (25% soln.) 1.38 g/cm3 (35% soln.) 1.46 g/cm3 (61% soln.) |
| Melting point | c. 19 °C (66 °F; 292 K) (60–70% solution) < −30 °C (−22 °F; 243 K) (35% solution) |
| Boiling point | 108.5 °C (227.3 °F; 381.6 K) (decomposes) |
| miscible | |
| Acidity (pKa) | 1.92[1] |
Refractive index (nD)
|
1.3465 |
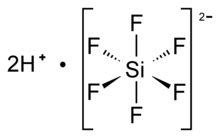
| Structure | |
| Octahedral SiF62− | |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
430 mg/kg (oral, rat) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
Hexafluorotitanic acid Hexafluorozirconic acid |
Other cations
|
Ammonium hexafluorosilicate |
Related compounds
|
Hexafluorophosphoric acid Fluoroboric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.
Hexafluorosilicic acid is produced naturally on a large scale in volcanoes.[2][3] It is manufactured as a coproduct in the production of phosphate fertilizers. The resulting hexafluorosilicic acid is almost exclusively consumed as a precursor to aluminum trifluoride and synthetic cryolite, which are used in aluminium processing. Salts derived from hexafluorosilicic acid are called hexafluorosilicates.
Structure
[edit]
Hexafluorosilicic acid has been crystallized as various hydrates. These include (H5O2)2SiF6, the more complicated (H5O2)2SiF6·2H2O, and (H5O2)(H7O3)SiF6·4.5H2O. In all of these salts, the octahedral hexafluorosilicate anion is hydrogen bonded to the cations.[4]
Aqueous solutions of hexafluorosilicic acid are often described as H
2SiF
6.
Production and principal reactions
[edit]Hexafluorosilicic acid is produced commercially from fluoride-containing minerals that also contain silicates. Specifically, apatite and fluorapatite are treated with sulfuric acid to give phosphoric acid, a precursor to several water-soluble fertilizers. This is called the wet phosphoric acid process.[5] As a by-product, approximately 50 kg of hexafluorosilicic acid is produced per tonne of HF owing to reactions involving silica-containing mineral impurities.[6]: 3
Some of the hydrogen fluoride (HF) produced during this process in turn reacts with silicon dioxide (SiO2) impurities, which are unavoidable constituents of the mineral feedstock, to give silicon tetrafluoride. Thus formed, the silicon tetrafluoride reacts further with HF.[citation needed] The net process can be described as:[7][page needed]
- 6 HF + SiO2 → SiF2−6 + 2 H3O+
Hexafluorosilicic acid can also be produced by treating silicon tetrafluoride with hydrofluoric acid.[7]
Reactions
[edit]Hexafluorosilic acid is only stable in hydrogen fluoride or acidic aqueous solutions. In any other circumstance, it acts as a source of hydrofluoric acid. Thus, for example, hexafluorosilicic acid pure or in oleum solution evolves silicon tetrafluoride until the residual hydrogen fluoride re-establishes equilibrium:[7]
- H2SiF6 ⇌ 2 HF(l) + SiF4(g)
In alkaline-to-neutral aqueous solutions, hexafluorosilicic acid readily hydrolyzes to fluoride anions and amorphous, hydrated silica ("SiO2"). Strong bases give fluorosilicate salts at first, but any stoichiometric excess begins hydrolysis.[7] At the concentrations usually used for water fluoridation, 99% hydrolysis occurs:[6][8]
- SiF2−
6 + 2 H2O → 6 F− + SiO2 + 4 H+
Alkali and alkaline earth salts
[edit]Neutralization of solutions of hexafluorosilicic acid with alkali metal bases produces the corresponding alkali metal fluorosilicate salts:
- H2SiF6 + 2 NaOH → Na2SiF6 + 2 H2O
The resulting salt Na2SiF6 is mainly used in water fluoridation. Related ammonium and barium salts are produced similarly for other applications. At room temperature 15-30% concentrated hexafluorosilicic acid undergoes similar reactions with chlorides, hydroxides, and carbonates of alkali and alkaline earth metals.[9]
Sodium hexafluorosilicate for instance may be produced by treating sodium chloride (NaCl) by hexafluorosilicic acid:[6]: 3 [10]: 7
- 2NaCl + H2SiF6 Na2SiF6↓ + 2 HCl
- BaCl2 + H2SiF6 BaSiF6↓ + 2 HCl
Heating sodium hexafluorosilicate gives silicon tetrafluoride:[10]: 8
- Na2SiF6 SiF4 + 2 NaF
Uses
[edit]The majority of the hexafluorosilicic acid is converted to aluminium fluoride and synthetic cryolite. These materials are central to the conversion of aluminium ore into aluminium metal. The conversion to aluminium trifluoride is described as:[7]
- H2SiF6 + Al2O3 → 2 AlF3 + SiO2 + H2O
Hexafluorosilicic acid is also converted to a variety of useful hexafluorosilicate salts. The potassium salt, Potassium fluorosilicate, is used in the production of porcelains, the magnesium salt for hardened concretes and as an insecticide, and the barium salts for phosphors.
Hexafluorosilicic acid and the salts are used as wood preservation agents.[11]
Lead refining
[edit]Hexafluorosilicic acid is also used as an electrolyte in the Betts electrolytic process for refining lead.
Rust removers
[edit]Hexafluorosilicic acid (identified as hydrofluorosilicic acid on the label) along with oxalic acid are the active ingredients used in Iron Out rust-removing cleaning products, which are essentially varieties of laundry sour.
Niche applications
[edit]H2SiF6 is a specialized reagent in organic synthesis for cleaving Si–O bonds of silyl ethers. It is more reactive for this purpose than HF. It reacts faster with t-butyldimethysilyl (TBDMS) ethers than triisopropylsilyl (TIPS) ethers.[12]
Treating concrete
[edit]The application of hexafluorosilica acid to a calcium rich surface such as concrete will give that surface some resistance to acid attack.[13]
- CaCO3 + H2O → Ca2+ + 2 OH− + CO2
- H2SiF6 → 2 H+ + SiF2−
6 - SiF2−
6 + 2 H2O → 6 F− + SiO2 + 4 H+ - Ca2+ + 2 F− → CaF2
Calcium fluoride (CaF2) is an insoluble solid that is acid resistant.
Natural salts
[edit]Some rare minerals, encountered either within volcanic or coal-fire fumaroles, are salts of the hexafluorosilicic acid. Examples include ammonium hexafluorosilicate that naturally occurs as two polymorphs: cryptohalite and bararite.[14][15][16]
Safety
[edit]Hexafluorosilicic acid can release hydrogen fluoride (HF) when evaporated, so it has similar risks. Inhalation of the vapors may cause lung edema. Like hydrogen fluoride, it attacks glass and stoneware.[17] The LD50 value of hexafluorosilicic acid is 430 mg/kg.[6]
See also
[edit]References
[edit]- ^ Perrin, D. D., ed. (1982) [1969]. Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). Entry 91. ISBN 0-08-029214-3. LCCN 82-16524.
- ^ Palache, C., Berman, H., and Frondel, C. (1951) Dana’s System of Mineralogy, Volume II: Halides, Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates, etc. John Wiley and Sons, Inc., New York, 7th edition.
- ^ Anthony, J.W., Bideaux, R.A., Bladh, K.W., and Nichols, M.C. (1997) Handbook of Mineralogy, Volume III: Halides, Hydroxides, Oxides. Mineral Data Publishing, Tucson.
- ^ a b Mootz, D.; Oellers, E.-J. (1988). "The Crystalline Hydrates of Hexafluorosilicic Acid: A Combined Phase-Analytical and Structural Study". Zeitschrift für anorganische und allgemeine Chemie. 559: 27–39. doi:10.1002/zaac.19885590103.
- ^ USGS. Fluorspar.
- ^ a b c d "Sodium Hexafluorosilicate [CASRN 16893-85-9] and Fluorosilicic Acid [CASRN 16961-83-4] Review of Toxicological Literature" (PDF). National Toxicology Program (U.S.). Archived (PDF) from the original on 22 October 2012. Retrieved 13 July 2017.
- ^ a b c d e Aigueperse, J.; Mollard, P.; Devilliers, D.; Chemla, M.; Faron, R.; Romano, R.; Cuer, J. P. (2005). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307. ISBN 978-3527306732.
- ^ Finney, William F.; Wilson, Erin; Callender, Andrew; Morris, Michael D.; Beck, Larry W. (2006). "Reexamination of Hexafluorosilicate Hydrolysis by 19F NMR and pH Measurement". Environ. Sci. Technol. 40 (8): 2572–2577. Bibcode:2006EnST...40.2572F. doi:10.1021/es052295s. PMID 16683594.
- ^ Hoffman CJ, Gutowsky HS, Schumb WC, Breck DW (1953). Silicon Tetrafluoride. Inorganic Syntheses. Vol. 4. pp. 147–8. doi:10.1002/9780470132357.ch47.
- ^ a b Us Granted A345458, Keith, C. Hansen & L. Yaws, Carl, "Patent Silicon tetrafluoride generation", published January 3, 1982, issued 1982
- ^ Carsten Mai, Holger Militz (2004). "Modification of wood with silicon compounds. inorganic silicon compounds and sol-gel systems: a review". Wood Science and Technology. 37 (5): 339. doi:10.1007/s00226-003-0205-5. S2CID 9672269.
- ^ Pilcher, A. S.; DeShong, P. (2001). "Fluorosilicic Acid". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rf013. ISBN 0471936235.
- ^ Properties of Concrete by A M Neville
- ^ "Cryptohalite".
- ^ "Bararite".
- ^ Kruszewski, Łukasz; Fabiańska, Monika J.; Segit, Tomasz; Kusy, Danuta; Motyliński, Rafał; Ciesielczuk, Justyna; Deput, Ewa (2020). "Carbon‑nitrogen compounds, alcohols, mercaptans, monoterpenes, acetates, aldehydes, ketones, SF6, PH3, and other fire gases in coal-mining waste heaps of Upper Silesian Coal Basin (Poland) – a re-investigation by means of in situ FTIR external database approach". Science of the Total Environment. 698: 134274. Bibcode:2020ScTEn.698m4274K. doi:10.1016/j.scitotenv.2019.134274. PMID 31509784. S2CID 202563638.
- ^ "Fluorosilicic Acid – International Chemical Safety Cards". NIOSH. Retrieved 2015-03-10.

