Riley oxidation
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
| Riley oxidation | |
|---|---|
| Named after | Harry Lister Riley |
| Reaction type | Organic redox reaction |
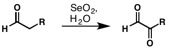
The Riley oxidation is a selenium dioxide-mediated oxidation of methylene groups adjacent to carbonyls. It was first reported by Harry Lister Riley and co-workers in 1932.[1] In the decade that ensued, selenium-mediated oxidation rapidly expanded in use, and in 1939, Andre Guillemonat and co-workers disclosed the selenium dioxide-mediated oxidation of olefins at the allylic position.[2] Today, selenium-dioxide-mediated oxidation of methylene groups to alpha ketones and at the allylic position of olefins is known as the Riley Oxidation.[3]

Mechanism
[edit]The mechanism of oxidation of -CH2C(O)R group by SeO2 has been well investigated.[4][5][6][7] The oxidation of carbonyl alpha methylene positions begins with attack by the enol tautomer at the electrophilic selenium center. Following rearrangement and loss of water, a second equivalent of water attacks the alpha position. Selenic acid is liberated in the final step to give the 1,2-dicarbonyl product.

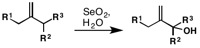
Allylic oxidation using selenium-dioxide proceeds via an ene reaction at the electrophilic selenium center. A 2,3-sigmatropic shift, proceeding through an envelope-like transition state, gives the allylselenite ester, which upon hydrolysis gives the allylic alcohol. The (E)- orientation about the double bond, a consequence of the envelope-like transition state, is observed in the penultimate ester formation, is retained during the hydrolysis step giving the (E)-allylic alcohol product.[4][8]

Scope
[edit]The Riley Oxidation is amenable to a variety of carbonyl and olefinic systems with a high degree of regiocontrol based on the substitution pattern of the given system.
Ketones with two available α-methylene positions react more quickly at the least hindered position.:[1]
Allylic oxidation can be predicted by the substitution pattern on the olefin. In the case of 1,2-disubstituted olefins, reaction rates follow CH > CH2 > CH3:

Geminally-substituted olefins react in the same order of reaction rates as above:[2]

Trisubstituted alkenes experience reactivity at the more substituted end of the double bond. The order of reactivity follows that CH2 > CH3 > CH:

Due to the rearrangement of the double bond, terminal olefins tend to give primary allylic alcohols:
Cyclic alkenes prefer to undergo allylic oxidation within the ring, rather than the allylic position at the sidechain. In bridged ring systems, Bredt’s rule is followed and bridgehead positions are not oxidized:

Applications
[edit]In their strychnine total synthesis, R.B. Woodward and co-workers leveraged the Riley Oxidation to attain the trans-glyoxal. Epimerization of the alpha hydrogen led to cis-glyoxal, which spontaneously underwent cyclization with the secondary amine to yield dehydrostryninone.[9]

Selenium-dioxide mediated oxidation was used in the synthesis of the diterpenoid ryanodol.[10]

Selenium dioxide mediated allylic oxidation to access ingenol.[11]

References
[edit]- ^ a b Riley, Harry Lister; Morley, John Frederick; Friend, Norman Alfred Child (1932-01-01). "255. Selenium dioxide, a new oxidising agent. Part I. Its reaction with aldehydes and ketones". Journal of the Chemical Society (Resumed): 1875–1883. doi:10.1039/jr9320001875. ISSN 0368-1769.
- ^ a b Guillemonat (1939). "Oxidation of ethylenic hydrocarbons using selenium dioxide".
- ^ Kurti, Laszlo (29 September 2005). Strategic Applications of Named Reactions in Organic Synthesis. Elsevier Science. pp. 380–381. ISBN 978-0-12-429785-2.
- ^ a b Trachtenberg, Edward N.; Nelson, Charles H.; Carver, Jane R. (1970-05-01). "Mechanism of selenium dioxide oxidation of olefins". The Journal of Organic Chemistry. 35 (5): 1653–1658. doi:10.1021/jo00830a083. ISSN 0022-3263.
- ^ Sharpless, Karl Barry; Gordon, Kenneth M. (1976-01-01). "Selenium dioxide oxidation of ketones and aldehydes. Evidence for the intermediacy of .beta.-ketoseleninic". Journal of the American Chemical Society. 98 (1): 300–301. doi:10.1021/ja00417a083. ISSN 0002-7863.
- ^ Warpehoski, M. A.; Chabaud, B.; Sharpless, Karl Barry (1982-07-01). "Selenium dioxide oxidation of endocyclic olefins. Evidence for a dissociation-recombination pathway". The Journal of Organic Chemistry. 47 (15): 2897–2900. doi:10.1021/jo00136a017. ISSN 0022-3263.
- ^ Shafer, Cynthia M.; Morse, Daniel I.; Molinski, Tadeusz F. (1996). "Mechanism of SeO2 promoted oxidative rearrangement of 2-substituted oxazolines to dihydrooxazinones: Isotopic labeling and kinetic studies". Tetrahedron. 52 (46): 14475–14486. doi:10.1016/0040-4020(96)00902-7.
- ^ Stephenson, L. M.; Speth, D. R. (1979). "Mechanism of allylic hydroxylation by selenium dioxide". The Journal of Organic Chemistry. 44 (25): 4683–4689. doi:10.1021/jo00393a045.
- ^ Woodward, R. B.; Cava, Michael P.; Ollis, W. D.; Hunger, A.; Daeniker, H. U.; Schenker, K. (1954-09-01). "The Total Synthesis of Strychnine". Journal of the American Chemical Society. 76 (18): 4749–4751. doi:10.1021/ja01647a088. ISSN 0002-7863. PMID 13305562. S2CID 42677858.
- ^ Chuang, Kangway V.; Xu, Chen; Reisman, Sarah E. (2016-08-26). "A 15-step synthesis of (+)-ryanodol". Science. 353 (6302): 912–915. Bibcode:2016Sci...353..912C. doi:10.1126/science.aag1028. ISSN 0036-8075. PMC 5505075. PMID 27563092.
- ^ Jørgensen, Lars; McKerrall, Steven J.; Kuttruff, Christian A.; Ungeheuer, Felix; Felding, Jakob; Baran, Phil S. (2013-08-23). "14-Step Synthesis of (+)-Ingenol from (+)-3-Carene". Science. 341 (6148): 878–882. Bibcode:2013Sci...341..878J. doi:10.1126/science.1241606. ISSN 0036-8075. PMID 23907534. S2CID 26998997.
