From Wikipedia, the free encyclopedia
Chemical compound
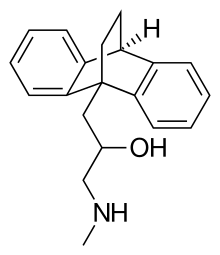
Oxaprotiline (developmental code name C 49-802 BDA ), also known as hydroxymaprotiline , is a norepinephrine reuptake inhibitor belonging to the tetracyclic antidepressant (TeCA) family and is related to maprotiline . Though investigated as an antidepressant ,[ 1]
Dextroprotiline acts as a potent norepinephrine reuptake inhibitor [ 2] [ 3] H1 receptor antagonist ,[ 4] α1 -adrenergic receptor antagonist .[ 2] [ 5] affinity for the serotonin transporter ,[ 2] dopamine transporter , α2 -adrenergic receptor ,[ 2] [ 5] muscarinic acetylcholine receptors .[ 5] antagonistic effects on the 5-HT2 , 5-HT7 , or D2 receptors like its relative maprotiline is unclear.
Levoprotiline acts as a selective H1 receptor antagonist , with no affinity for adrenaline , dopamine , muscarinic acetylcholine , or serotonin receptors , or any of the monoamine transporters .[ 2] [ 3] [ 4]
Oxaprotiline is a racemic compound composed of two isomers , R (−)- or levo - oxaprotiline (levoprotiline ; CGP-12,103-A ), and S (+)- or dextro- oxaprotiline (dextroprotiline ; CGP-12,104-A ). Both enantiomers are active, with the levo- form acting as an antihistamine and the dextro- form having an additional pharmacology (see above ), but with both unexpectedly still retaining antidepressant effects.[ 6]
^ Giedke H, Gaertner H, Breyer-Pfaff U, Rein W, Axmann D (1986). "Amitriptyline and oxaprotiline in the treatment of hospitalized depressive patients. Clinical aspects, psychophysiology, and drug plasma levels" . European Archives of Psychiatry and Neurological Sciences . 235 (6): 329–338. doi :10.1007/bf00381001 . PMID 3527706 . S2CID 24152419 . ^ a b c d e Waldmeier PC, Baumann PA, Hauser K, Maitre L, Storni A (June 1982). "Oxaprotiline, a noradrenaline uptake inhibitor with an active and an inactive enantiomer". Biochemical Pharmacology 31 (12): 2169–76. doi :10.1016/0006-2952(82)90510-X . PMID 7115436 . ^ a b Reimann IW, Firkusny L, Antonin KH, Bieck PR (1993). "Oxaprotiline: enantioselective noradrenaline uptake inhibition indicated by intravenous amine pressor tests but not alpha 2-adrenoceptor binding to intact platelets in man". European Journal of Clinical Pharmacology . 44 (1): 93–5. doi :10.1007/BF00315288 . PMID 8382162 . S2CID 22691825 . ^ a b Noguchi S, Inukai T, Kuno T, Tanaka C (June 1992). "The suppression of olfactory bulbectomy-induced muricide by antidepressants and antihistamines via histamine H1 receptor blocking". Physiology & Behavior . 51 (6): 1123–7. doi :10.1016/0031-9384(92)90297-F . PMID 1353628 . S2CID 29562845 . ^ a b c Richelson E, Nelson A (July 1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro" . The Journal of Pharmacology and Experimental Therapeutics . 230 (1): 94–102. PMID 6086881 . ^ Noguchi S, Fukuda Y, Inukai T (May 1992). "Possible contributory role of the central histaminergic system in the forced swimming model". Arzneimittel-Forschung . 42 (5): 611–3. PMID 1530672 .
SSRIs Tooltip Selective serotonin reuptake inhibitors SNRIs Tooltip Serotonin–norepinephrine reuptake inhibitors NRIs Tooltip Norepinephrine reuptake inhibitors NDRIs Tooltip Norepinephrine–dopamine reuptake inhibitors NaSSAs Tooltip Noradrenergic and specific serotonergic antidepressants SARIs Tooltip Serotonin antagonist and reuptake inhibitors SMS Tooltip Serotonin modulator and stimulators Others
TCAs Tooltip Tricyclic antidepressants TeCAs Tooltip Tetracyclic antidepressants Others
Non-selective MAOA Tooltip Monoamine oxidase A -selectiveMAOB Tooltip Monoamine oxidase B -selective
α1
Agonists Antagonists
Abanoquil Ajmalicine Alfuzosin Anisodamine Anisodine Atiprosin Atypical antipsychotics (e.g., brexpiprazole , clozapine , olanzapine , quetiapine , risperidone )Benoxathian Beta blockers (e.g., adimolol , amosulalol , arotinolol , carvedilol , eugenodilol , labetalol )Buflomedil Bunazosin Corynanthine Dapiprazole Domesticine Doxazosin Ergolines (e.g., acetergamine , ergotamine , dihydroergotamine , lisuride , nicergoline , terguride )Etoperidone Fenspiride Hydroxyzine Indoramin Ketanserin L-765,314 mCPP Mepiprazole Metazosin Monatepil Moxisylyte Naftopidil Nantenine Neldazosin Niaprazine Niguldipine Pardoprunox Pelanserin Perlapine Phendioxan Phenoxybenzamine Phentolamine Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Piperoxan Prazosin Quinazosin Quinidine Silodosin Spegatrine Spiperone Talipexole Tamsulosin Terazosin Tiodazosin Tolazoline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , imipramine , trimipramine )Trimazosin Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Urapidil WB-4101 Zolertine
α2
Agonists Antagonists
1-PP Adimolol Amesergide Aptazapine Atipamezole Atypical antipsychotics (e.g., asenapine , brexpiprazole , clozapine , lurasidone , olanzapine , paliperidone , quetiapine , risperidone , zotepine )Azapirones (e.g., buspirone , gepirone , ipsapirone , tandospirone )BRL-44408 Buflomedil Cirazoline Efaroxan Esmirtazapine Fenmetozole Fluparoxan Idazoxan Ketanserin Lisuride mCPP Mianserin Mirtazapine NAN-190 Pardoprunox Phentolamine Phenoxybenzamine Piperoxan Piribedil Rauwolscine Rotigotine Setiptiline Spegatrine Spiroxatrine Sunepitron Terguride Tolazoline Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Yohimbine
β
H1
Agonists Antagonists
Others: Atypical antipsychotics (e.g., aripiprazole , asenapine , brexpiprazole , brilaroxazine , clozapine , iloperidone , olanzapine , paliperidone , quetiapine , risperidone , ziprasidone , zotepine )Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Tetracyclic antidepressants (e.g., amoxapine , loxapine , maprotiline , mianserin , mirtazapine , oxaprotiline )Tricyclic antidepressants (e.g., amitriptyline , butriptyline , clomipramine , desipramine , dosulepin (dothiepin) , doxepin , imipramine , iprindole , lofepramine , nortriptyline , protriptyline , trimipramine )Typical antipsychotics (e.g., chlorpromazine , flupenthixol , fluphenazine , loxapine , perphenazine , prochlorperazine , thioridazine , thiothixene )
H2
H3
H4
DAT Tooltip Dopamine transporter (DRIs Tooltip Dopamine reuptake inhibitors )
NET Tooltip Norepinephrine transporter (NRIs Tooltip Norepinephrine reuptake inhibitors )
Others: Antihistamines (e.g., brompheniramine , chlorphenamine , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., ketamine , phencyclidine )Dopexamine Ephenidine Ginkgo biloba Indeloxazine Nefazodone Opioids (e.g., desmetramadol , methadone , pethidine (meperidine) , tapentadol , tramadol , levorphanol )
SERT Tooltip Serotonin transporter (SRIs Tooltip Serotonin reuptake inhibitors )
Others: A-80426 Amoxapine Antihistamines (e.g., brompheniramine , chlorphenamine , dimenhydrinate , diphenhydramine , mepyramine (pyrilamine) , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., 3-MeO-PCP , esketamine , ketamine , methoxetamine , phencyclidine )Cyclobenzaprine Delucemine Dextromethorphan Dextrorphan Efavirenz Hypidone Medifoxamine Mesembrine Mifepristone MIN-117 (WF-516) N-Me-5-HT Opioids (e.g., dextropropoxyphene , methadone , pethidine (meperidine) , levorphanol , tapentadol , tramadol )Roxindole
VMATs Tooltip Vesicular monoamine transporters Others