Electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons.[1][2][3] This includes most soluble salts, acids, and bases, dissolved in a polar solvent like water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent.[4] Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.[5][6]
Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes.[clarification needed] Electrolyte solutions can also result from the dissolution of some biological (e.g., DNA, polypeptides) or synthetic polymers (e.g., polystyrene sulfonate), termed "polyelectrolytes", which contain charged functional groups. A substance that dissociates into ions in solution or in the melt acquires the capacity to conduct electricity. Sodium, potassium, chloride, calcium, magnesium, and phosphate in a liquid phase are examples of electrolytes.
In medicine, electrolyte replacement is needed when a person has prolonged vomiting or diarrhea, and as a response to sweating due to strenuous athletic activity. Commercial electrolyte solutions are available, particularly for sick children (such as oral rehydration solution, Suero Oral, or Pedialyte) and athletes (sports drinks). Electrolyte monitoring is important in the treatment of anorexia and bulimia.
In science, electrolytes are one of the main components of electrochemical cells.[2]
In clinical medicine, mentions of electrolytes usually refer metonymically to the ions, and (especially) to their concentrations (in blood, serum, urine, or other fluids). Thus, mentions of electrolyte levels usually refer to the various ion concentrations, not to the fluid volumes.
Etymology
[edit]The word electrolyte derives from Ancient Greek ήλεκτρο- (ēlectro-), prefix originally meaning amber but in modern contexts related to electricity, and λυτός (lytos), meaning "able to be untied or loosened".[citation needed]
History
[edit]
In his 1884 dissertation, Svante Arrhenius put forth his explanation of solid crystalline salts disassociating into paired charged particles when dissolved, for which he won the 1903 Nobel Prize in Chemistry.[7][8][9][10] Arrhenius's explanation was that in forming a solution, the salt dissociates into charged particles, to which Michael Faraday (1791-1867) had given the name "ions" many years earlier. Faraday's belief had been that ions were produced in the process of electrolysis. Arrhenius proposed that, even in the absence of an electric current, solutions of salts contained ions. He thus proposed that chemical reactions in solution were reactions between ions.[8][9][10]
Shortly after Arrhenius's hypothesis of ions, Franz Hofmeister and Siegmund Lewith[11][12][13] found that different ion types displayed different effects on such things as the solubility of proteins. A consistent ordering of these different ions on the magnitude of their effect arises consistently in many other systems as well. This has since become known as the Hofmeister series.
While the origins of these effects are not abundantly clear and have been debated throughout the past century, it has been suggested that the charge density of these ions is important[14] and might actually have explanations originating from the work of Charles-Augustin de Coulomb over 200 years ago.
Formation
[edit]Electrolyte solutions are normally formed when salt is placed into a solvent such as water and the individual components dissociate due to the thermodynamic interactions between solvent and solute molecules, in a process called "solvation". For example, when table salt (sodium chloride), NaCl, is placed in water, the salt (a solid) dissolves into its component ions, according to the dissociation reaction:[citation needed]
- NaCl(s) → Na+(aq) + Cl−(aq)
It is also possible for substances to react with water, producing ions. For example, carbon dioxide gas dissolves in water to produce a solution that contains hydronium, carbonate, and hydrogen carbonate ions.[citation needed]
Molten salts can also be electrolytes as, for example, when sodium chloride is molten, the liquid conducts electricity. In particular, ionic liquids, which are molten salts with melting points below 100 °C,[15] are a type of highly conductive non-aqueous electrolytes and thus have found more and more applications in fuel cells and batteries.[16]
An electrolyte in a solution may be described as "concentrated" if it has a high concentration of ions, or "dilute" if it has a low concentration. If a high proportion of the solute dissociates to form free ions, the electrolyte is strong; if most of the solute does not dissociate, the electrolyte is weak. The properties of electrolytes may be exploited using electrolysis to extract constituent elements and compounds contained within the solution.[citation needed]
Alkaline earth metals form hydroxides that are strong electrolytes with limited solubility in water, due to the strong attraction between their constituent ions. This limits their application to situations where high solubility is required.[17]
In 2021, researchers have found that electrolyte can "substantially facilitate electrochemical corrosion studies in less conductive media".[18]
Physiological importance
[edit]In physiology, the primary ions of electrolytes are sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), hydrogen phosphate (HPO42−), and hydrogen carbonate (HCO3−).[19][failed verification] The electric charge symbols of plus (+) and minus (−) indicate that the substance is ionic in nature and has an imbalanced distribution of electrons, the result of chemical dissociation. Sodium is the main electrolyte found in extracellular fluid and potassium is the main intracellular electrolyte;[20] both are involved in fluid balance and blood pressure control.[21]
All known multicellular lifeforms require a subtle and complex electrolyte balance between the intracellular and extracellular environments.[19] In particular, the maintenance of precise osmotic gradients of electrolytes is important. Such gradients affect and regulate the hydration of the body as well as blood pH, and are critical for nerve and muscle function. Various mechanisms exist in living species that keep the concentrations of different electrolytes under tight control.[22]
Both muscle tissue and neurons are considered electric tissues of the body. Muscles and neurons are activated by electrolyte activity between the extracellular fluid or interstitial fluid, and intracellular fluid. Electrolytes may enter or leave the cell membrane through specialized protein structures embedded in the plasma membrane called "ion channels". For example, muscle contraction is dependent upon the presence of calcium (Ca2+), sodium (Na+), and potassium (K+). Without sufficient levels of these key electrolytes, muscle weakness or severe muscle contractions may occur.[citation needed][23]
Electrolyte balance is maintained by oral, or in emergencies, intravenous (IV) intake of electrolyte-containing substances, and is regulated by hormones, in general with the kidneys flushing out excess levels. In humans, electrolyte homeostasis is regulated by hormones such as antidiuretic hormones, aldosterone and parathyroid hormones. Serious electrolyte disturbances, such as dehydration and overhydration, may lead to cardiac and neurological complications and, unless they are rapidly resolved, will result in a medical emergency.
Measurement
[edit]Measurement of electrolytes is a commonly performed diagnostic procedure, performed via blood testing with ion-selective electrodes or urinalysis by medical technologists. The interpretation of these values is somewhat meaningless without analysis of the clinical history and is often impossible without parallel measurements of renal function. The electrolytes measured most often are sodium and potassium. Chloride levels are rarely measured except for arterial blood gas interpretations since they are inherently linked to sodium levels. One important test conducted on urine is the specific gravity test to determine the occurrence of an electrolyte imbalance.[citation needed]
Rehydration
[edit]According to a study paid for by the Gatorade Sports Science Institute, electrolyte drinks containing sodium and potassium salts replenish the body's water and electrolyte concentrations after dehydration caused by exercise, excessive alcohol consumption, diaphoresis (heavy sweating), diarrhea, vomiting, intoxication or starvation; the study says that athletes exercising in extreme conditions (for three or more hours continuously, e.g. a marathon or triathlon) who do not consume electrolytes risk dehydration (or hyponatremia).[24][needs independent confirmation]
A home-made electrolyte drink can be made by using water, sugar and salt in precise proportions.[25] It is important to include glucose (sugar) to utilise the co-transport mechanism of sodium and glucose. Commercial preparations are also available[26] for both human and veterinary use.
Electrolytes are commonly found in fruit juices, sports drinks, milk, nuts, and many fruits and vegetables (whole or in juice form) (e.g., potatoes, avocados).
Electrochemistry
[edit]When electrodes are placed in an electrolyte and a voltage is applied, the electrolyte will conduct electricity. Lone electrons normally cannot pass through the electrolyte; instead, a chemical reaction occurs at the cathode, providing electrons to the electrolyte. Another reaction occurs at the anode, consuming electrons from the electrolyte. As a result, a negative charge cloud develops in the electrolyte around the cathode, and a positive charge develops around the anode. The ions in the electrolyte neutralize these charges, enabling the electrons to keep flowing and the reactions to continue.[citation needed]
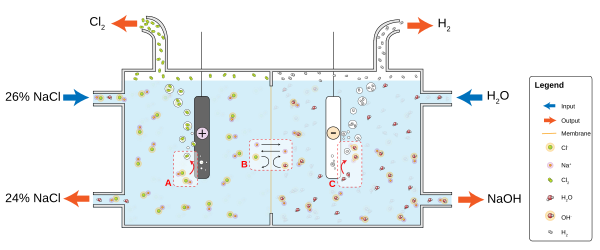
For example, in a solution of ordinary table salt (sodium chloride, NaCl) in water, the cathode reaction will be
- 2 H2O + 2e− → 2 OH− + H2
and hydrogen gas will bubble up; the anode reaction is
- 2 NaCl → 2 Na+ + Cl2 + 2e−
and chlorine gas will be liberated into solution where it reacts with the sodium and hydroxyl ions to produce sodium hypochlorite - household bleach. The positively charged sodium ions Na+ will react toward the cathode, neutralizing the negative charge of OH− there, and the negatively charged hydroxide ions OH− will react toward the anode, neutralizing the positive charge of Na+ there. Without the ions from the electrolyte, the charges around the electrode would slow down continued electron flow; diffusion of H+ and OH− through water to the other electrode takes longer than movement of the much more prevalent salt ions. Electrolytes dissociate in water because water molecules are dipoles and the dipoles orient in an energetically favorable manner to solvate the ions.
In other systems, the electrode reactions can involve the metals of the electrodes as well as the ions of the electrolyte.
Electrolytic conductors are used in electronic devices where the chemical reaction at a metal-electrolyte interface yields useful effects.
- In batteries, two materials with different electron affinities are used as electrodes; electrons flow from one electrode to the other outside of the battery, while inside the battery the circuit is closed by the electrolyte's ions. Here, the electrode reactions convert chemical energy to electrical energy.[27]
- In some fuel cells, a solid electrolyte or proton conductor connects the plates electrically while keeping the hydrogen and oxygen fuel gases separated.[28]
- In electroplating tanks, the electrolyte simultaneously deposits metal onto the object to be plated, and electrically connects that object in the circuit.
- In operation-hours gauges, two thin columns of mercury are separated by a small electrolyte-filled gap, and, as charge is passed through the device, the metal dissolves on one side and plates out on the other, causing the visible gap to slowly move along.
- In electrolytic capacitors the chemical effect is used to produce an extremely thin dielectric or insulating coating, while the electrolyte layer behaves as one capacitor plate.
- In some hygrometers the humidity of air is sensed by measuring the conductivity of a nearly dry electrolyte.
- Hot, softened glass is an electrolytic conductor, and some glass manufacturers keep the glass molten by passing a large current through it.
Solid electrolytes
[edit]Solid electrolytes can be mostly divided into four groups described below.
Gel electrolytes
[edit]Gel electrolytes – closely resemble liquid electrolytes. In essence, they are liquids in a flexible lattice framework. Various additives are often applied to increase the conductivity of such systems.[27][29]
Ceramic electrolytes
[edit]Solid ceramic electrolytes – ions migrate through the ceramic phase by means of vacancies or interstitials within the lattice. There are also glassy-ceramic electrolytes.
Polymer electrolytes
[edit]Dry polymer electrolytes – differ from liquid and gel electrolytes in the sense that salt is dissolved directly into the solid medium. Usually it is a relatively high-dielectric constant polymer (PEO, PMMA, PAN, polyphosphazenes, siloxanes, etc.) and a salt with low lattice energy. In order to increase the mechanical strength and conductivity of such electrolytes, very often composites are made, and inert ceramic phase is introduced. There are two major classes of such electrolytes: polymer-in-ceramic, and ceramic-in-polymer.[30][31][32]
Organic plastic electrolytes
[edit]Organic ionic plastic crystals – are a type organic salts exhibiting mesophases (i.e. a state of matter intermediate between liquid and solid), in which mobile ions are orientationally or rotationally disordered while their centers are located at the ordered sites in the crystal structure.[28] They have various forms of disorder due to one or more solid–solid phase transitions below the melting point and have therefore plastic properties and good mechanical flexibility as well as an improved electrode-electrolyte interfacial contact. In particular, protic organic ionic plastic crystals (POIPCs),[28] which are solid protic organic salts formed by proton transfer from a Brønsted acid to a Brønsted base and in essence are protic ionic liquids in the molten state, have found to be promising solid-state proton conductors for fuel cells. Examples include 1,2,4-triazolium perfluorobutanesulfonate[28] and imidazolium methanesulfonate.[33]
See also
[edit]- Electrochemical machining
- Elektrolytdatenbank Regensburg
- Ion transport number
- ITIES (interface between two immiscible electrolyte solutions)
- Salt bridge
- Strong electrolyte
- Supporting electrolyte (background electrolyte)
- VTPR
References
[edit]- ^ Enderby JE, Neilson GW (1 June 1981). "The structure of electrolyte solutions". Reports on Progress in Physics. 44 (6): 593–653. doi:10.1088/0034-4885/44/6/001. ISSN 0034-4885. S2CID 250852242. Archived from the original on 18 December 2021. Retrieved 18 December 2021.
- ^ a b Petrovic S (29 October 2020). Battery technology crash course : a concise introduction. Springer. ISBN 978-3-030-57269-3. OCLC 1202758685.
- ^ Winie T, Arof AK, Thomas S (18 February 2020). Polymer Electrolytes: Characterization Techniques and Energy Applications. John Wiley & Sons. ISBN 978-3-527-34200-6.
- ^ M Andreev, JJ de Pablo, A Chremos, J F Douglas (2018). "Influence of ion solvation on the properties of electrolyte solutions". The Journal of Physical Chemistry B. 122 (14): 4029–4034. doi:10.1021/acs.jpcb.8b00518. PMID 29611710.
- ^ Wilkins LW (2007). Fluids and Electrolytes. Lippincott Williams & Wilkins. ISBN 978-1-58255-923-0.
- ^ "electrolyte". National Cancer Institute. 2 February 2011. Archived from the original on 23 April 2018. Retrieved 18 December 2021.
- ^ "The Nobel Prize in Chemistry 1903". Archived from the original on 8 July 2018. Retrieved 5 January 2017.
- ^ a b Harris W, Levey J, eds. (1975). The New Columbia Encyclopedia (4th ed.). New York City: Columbia University. p. 155. ISBN 978-0-231035-729.
- ^ a b McHenry C, ed. (1992). The New Encyclopædia Britannica. Vol. 1 (15 ed.). Chicago: Encyclopædia Britannica, Inc. p. 587. Bibcode:1991neb..book.....G. ISBN 978-085-229553-3.
- ^ a b Cillispie C, ed. (1970). Dictionary of Scientific Biography (1 ed.). New York City: Charles Scribner's Sons. pp. 296–302. ISBN 978-0-684101-125.
- ^ Franz Hofmeister (1888). "Zur Lehre Von Der Wirkung Der Salze". Naunyn-Schmiedeberg's Arch. Pharmacol.
- ^ W. Kunz, J. Henle, B. W. Ninham (2004). "'Zur Lehre von der Wirkung der Salze' (about the science of the effect of salts): Franz Hofmeister's historical papers". Current Opinion in Colloid & Interface Science. 9 (1–2): 19–37. doi:10.1016/j.cocis.2004.05.005. Archived from the original on 20 January 2022. Retrieved 8 November 2021.
- ^ Gregory KP, Elliott GR, Robertson H, Kumar A, Wanless EJ, Webber GB, Craig VS, Andersson GG, Page AJ (2022). "Understanding specific ion effects and the Hofmeister series". Physical Chemistry Chemical Physics. 24 (21): 12682–12718. Bibcode:2022PCCP...2412682G. doi:10.1039/D2CP00847E. PMID 35543205.
- ^ Kasimir P. Gregory, Erica J. Wanless, Grant B. Webber, Vince S. J. Craig, Alister J. Page (2021). "The Electrostatic Origins of Specific Ion Effects: Quantifying the Hofmeister Series for Anions". Chem. Sci. 12 (45): 15007–15015. doi:10.1039/D1SC03568A. PMC 8612401. PMID 34976339. S2CID 244578563.
- ^ Shi J, Sun X, Chunhe Y, Gao Q, Li Y (2002). 离子液体研究进展 (PDF). 化学通报 (in Simplified Chinese) (4): 243. ISSN 0441-3776. Archived from the original (PDF) on 2 March 2017. Retrieved 1 March 2017.
- ^ Jiangshui Luo, Jin Hu, Wolfgang Saak, Rüdiger Beckhaus, Gunther Wittstock, Ivo F. J. Vankelecom, Carsten Agert, Olaf Conrad (2011). "Protic ionic liquid and ionic melts prepared from methanesulfonic acid and 1H-1,2,4-triazole as high temperature PEMFC electrolytes". Journal of Materials Chemistry. 21 (28): 10426–10436. doi:10.1039/C0JM04306K. S2CID 94400312.
- ^ Brown, Chemistry: The Central Science, 14th edition, pg. 680.
- ^ Matějovský L, Staš M, Dumská K, Pospíšil M, Macák J (1 January 2021). "Electrochemical corrosion tests in an environment of low-conductive ethanol-gasoline blends: Part 1 – Testing of supporting electrolytes". Journal of Electroanalytical Chemistry. 880: 114879. doi:10.1016/j.jelechem.2020.114879. ISSN 1572-6657. S2CID 229508133.
- ^ a b Alfarouk KO, Ahmed SB, Ahmed A, Elliott RL, Ibrahim ME, Ali HS, Wales CC, Nourwali I, Aljarbou AN, Bashir AH, Alhoufie ST, Alqahtani SS, Cardone RA, Fais S, Harguindey S, Reshkin SJ (7 April 2020). "The Interplay of Dysregulated pH and Electrolyte Imbalance in Cancer". Cancers. 12 (4): 898. doi:10.3390/cancers12040898. PMC 7226178. PMID 32272658.
- ^ Ye S(, Tang Z( (1986). 细胞膜钠泵及其临床意义. 上海医学 [Shanghai Medicine] (in Simplified Chinese) (1): 1. Archived from the original on 3 March 2017. Retrieved 3 March 2017.
- ^ Tu Z( (2004). 电解质紊乱对晚期肿瘤的治疗影响. 中华中西医杂志 [Chinese Magazine of Chinese and Western Medicine] (in Simplified Chinese) (10). 张定昌.
在正常人体内,钠离子占细胞外液阳离子总量的92%,钾离子占细胞内液阳离子总量的98%左右。钠、钾离子的相对平衡,维持着整个细胞的功能和结构的完整。钠、钾是人体内最主要的电解质成分...
- ^ Open Resources for Nursing, Ernstmeyer K, Christman E (2021), "Chapter 15 Fluids and Electrolytes", Nursing Fundamentals [Internet], Chippewa Valley Technical College, retrieved 28 February 2024
- ^ "Reproductive Consequences of Electrolyte Disturbances in Domestic Animals".
- ^ J, Estevez E, Baquero E, Mora-Rodriguez R (2008). "Anaerobic performance when rehydrating with water or commercially available sports drinks during prolonged exercise in the heat". Applied Physiology, Nutrition, and Metabolism. 33 (2): 290–298. doi:10.1139/H07-188. PMID 18347684.
- ^ "Rehydration drinks". Webmd.com. 28 April 2008. Archived from the original on 23 October 2008. Retrieved 25 December 2018.
- ^ "Oral Rehydration Salt Suppliers". Rehydrate.org. 7 October 2014. Archived from the original on 7 December 2014. Retrieved 4 December 2014.
- ^ a b Kamil Perzyna, Regina Borkowska, Jaroslaw Syzdek, Aldona Zalewska, Wladyslaw Wieczorek (2011). "The effect of additive of Lewis acid type on lithium–gel electrolyte characteristics". Electrochimica Acta. 57: 58–65. doi:10.1016/j.electacta.2011.06.014.
- ^ a b c d Jiangshui Luo, Annemette H. Jensen, Neil R. Brooks, Jeroen Sniekers, Martin Knipper, David Aili, Qingfeng Li, Bram Vanroy, Michael Wübbenhorst, Feng Yan, Luc Van Meervelt, Zhigang Shao, Jianhua Fang, Zheng-Hong Luo, Dirk E. De Vos, Koen Binnemans, Jan Fransaer (2015). "1,2,4-Triazolium perfluorobutanesulfonate as an archetypal pure protic organic ionic plastic crystal electrolyte for all-solid-state fuel cells". Energy & Environmental Science. 8 (4): 1276–1291. doi:10.1039/C4EE02280G. S2CID 84176511.
- ^ "The Roll-to-Roll Battery Revolution". Ev World. Archived from the original on 10 July 2011. Retrieved 20 August 2010.
- ^ Syzdek J, Borkowska R, Perzyna K, Tarascon JM, Wieczorek W (2007). "Novel composite polymeric electrolytes with surface-modified inorganic fillers". Journal of Power Sources. 173 (2): 712–720. Bibcode:2007JPS...173..712S. doi:10.1016/j.jpowsour.2007.05.061. ISSN 0378-7753.
- ^ Syzdek J, Armand M, Marcinek M, Zalewska A, Żukowska G, Wieczorek W (2010). "Detailed studies on the fillers modification and their influence on composite, poly(oxyethylene)-based polymeric electrolytes". Electrochimica Acta. 55 (4): 1314–1322. doi:10.1016/j.electacta.2009.04.025. ISSN 0013-4686.
- ^ Syzdek J, Armand M, Gizowska M, Marcinek M, Sasim E, Szafran M, Wieczorek W (2009). "Ceramic-in-polymer versus polymer-in-ceramic polymeric electrolytes—A novel approach". Journal of Power Sources. 194 (1): 66–72. Bibcode:2009JPS...194...66S. doi:10.1016/j.jpowsour.2009.01.070. ISSN 0378-7753.
- ^ Jiangshui Luo, Olaf Conrad, Ivo F. J. Vankelecom (2013). "Imidazolium methanesulfonate as a high temperature proton conductor". Journal of Materials Chemistry A. 1 (6): 2238–2247. doi:10.1039/C2TA00713D. S2CID 96622511.
External links
[edit] Media related to Electrolytes at Wikimedia Commons
Media related to Electrolytes at Wikimedia Commons- Friedman HL (1960). "Mayer's Ionic Solution Theory Applied to Electrolyte Mixtures". The Journal of Chemical Physics. 32 (4): 1134–1149. Bibcode:1960JChPh..32.1134F. doi:10.1063/1.1730863.
- Leaist DG, Lyons PA (1981). "Multicomponent diffusion of electrolytes with incomplete dissociation. Diffusion in a buffer solution". The Journal of Physical Chemistry. 85 (12): 1756–1762. doi:10.1021/j150612a033.
- Kaminsky M (1957). "Ion-solvent interaction and the viscosity of strong-electrolyte solutions". Discussions of the Faraday Society. 24: 171. doi:10.1039/DF9572400171.

