Bemethyl
 | |
| Clinical data | |
|---|---|
| Trade names | Metaprot, Antihot, Bemitil, Bemithylum, Bemactor |
| Other names | bemetil, bemithil, bemithyl, bemythyl, Metaproth, Metaprote, 2-benzilidazol-thioethyl, 2-ethylthiobenzimidazole hydrobromide, 2-ethylsulfanyl-1H-benzimidazole;hydrobromide |
| Routes of administration | Oral as (tablets or capsules) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
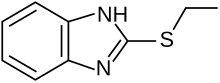
| Formula | C9H10N2S |
| Molar mass | 178.25 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Bemethyl, also commonly referred to in literature as bemitil, is a synthetic actoprotector which is also antihypoxant (combating conditions of hypoxia), antioxidant, and antimutagenic. Bemethyl is primarily classified as an actoprotector: a synthetic adaptogen with significant capacity to increase physical performance.[1]
It is approved for use in Ukraine as a dietary supplement. Bemethyl is commonly used in preparing for international competitions by Ukrainian national sport teams.[1]
Bemethyl is formulated as a hydrobromide salt. Its parent compound is 2-ethylsulfanyl-1H-benzimidazole.[2]
Research
[edit]Metabolic modulation
[edit]Bemethyl also has a positive effect on metabolic processes, allowing adaptation to conditions causing frequent hypoxia, and the obtained effect is long-lasting and also occurs after the end of dosage.[3]
Anti-hypoxant
[edit]Bemethyl has been shown to preserve both physical and mental capacity in high-altitude, low-oxygen environments, particularly by its effect in helping control excess serum levels of cholesterol and bilirubin, which are known to have negative effects especially during adjustment to high-altitude environments.[4]
Recovery from trauma
[edit]Bemethyl has also been shown to prevent permanent hearing loss and facilitate recovery of hearing after mine-explosion trauma, when treatment is initiated immediately after injury.[5]
Anti-mutagenic
[edit]In one study, bemethyl was shown to prevent the mutagenic effect of white asbestos in mice and in cultured human whole blood.[6]
A study using mice showed bemethyl to reduce mutation induced by certain mutagenic drugs.[7]
Another study using cells from human donors showed Bemethyl to be anticlastogenic (able to minimize chromosome breakages).[8]
Anti-asthenic
[edit]A study using mice established that both single and continuous dosing of bemethyl increases work capacity and accelerates rehabilitation after exhaustive exertion.[1]
Pharmacology
[edit]Pharmacodynamics
[edit]The mechanistic underpinnings of bemethyl's effects are not well understood, but some evidence implicate alterations in protein synthesis and glucose metabolism, in addition to antioxidant activity, as relevant mechanisms of action.[1]
Pharmacokinetics
[edit]Bemethyl resists metabolization and is long-lived, accumulating in tissues over course of treatment. In one study involving rats, long-term administration of Bemethyl was accompanied by a 1.38-fold increase in drug concentration in the brain, and a 1.68-fold increase in its concentration in skeletal muscles.[9]
History
[edit]Bemethyl was developed in the 1970s by the Department of Pharmacology of the St. Petersburg State Military Medical Academy under the direction of Professor Vladimir Vinogradov. Professor Vinogradov and his research team earned the USSR State Prize for this accomplishment.[1]
First used with Soviet cosmonauts, bemethyl was also used to prepare athletes of the USSR national team for the Moscow 1980 Olympic Games. In the 1990s, bemethyl saw use as a basic medicinal agent in many of the corps of the Soviet and Russian armies, including Soviet troops in Afghanistan, as bemethyl facilitated increased endurance for soldiers over long marches, as well as an enhanced work capacity and stability to hypoxia and high temperatures. Bemethyl was also used to enhance the physical and mental capacities of workers deployed in the wake of the 1986 Chernobyl disaster.[1]
Legal
[edit]In 2018, bemethyl was added to the Monitoring Program of the World Anti-Doping Agency. [10] As of 2021 it remains in the Monitoring Program, [11] and therefore is not on the Prohibited List; presently only being monitored to potentially detect patterns of misuse in sport. The WADA Monitoring Program is also recognized by the U.S. Anti-Doping Agency, [12] as bemethyl continues not to be a "banned substance" in sports.
See also
[edit]References
[edit]- ^ a b c d e f Oliynyk S, Oh S (September 2012). "The pharmacology of actoprotectors: practical application for improvement of mental and physical performance". Biomolecules & Therapeutics. 20 (5): 446–56. doi:10.4062/biomolther.2012.20.5.446. PMC 3762282. PMID 24009833.
- ^ "PubChem Compound Summary for CID 9816609 (Bemethyl)".
- ^ Zarubina IV, Nurmanbetova FN, Shabanov PD (August 2005). "Bemithyl potentiates the antioxidant effect of intermittent hypoxic training". Bulletin of Experimental Biology and Medicine. 140 (2): 190–3. doi:10.1007/s10517-005-0442-8. PMID 16282998. S2CID 1170824.
- ^ Mahnovsky VP (2001). Pharmacological Correction of the Human Functional State in High Altitude Conditions (PDF) (Report). Operational Medical Issues in Hypo- and Hyperbaric Conditions. Toronto, Canada: NATO Science and Technology Organization. pp. 6–1..6–15. ADPO 11064. Archived from the original (PDF) on March 5, 2017.
- ^ Salikhov K (2012-07-17). Challenges in Treating Combat Injuries. Xlibris Corporation. p. 299. ISBN 9781477156995.
- ^ Daugel'-Dauge NO, Durnev AD, Kulakova AV, Seredenin SB, Velichkovskiĭ BT (1995). "[Corpuscular mutagenesis and its prevention]". Vestnik Rossiiskoi Akademii Meditsinskikh Nauk (1): 29–38. PMID 7767114.
- ^ Seredenin SB, Bobkov I, Durnev AD, Dubovskaia OI (July 1986). "[Mutagenic and antimutagenic properties of bemitil]". Biulleten' Eksperimental'noi Biologii I Meditsiny. 102 (7): 76–9. PMID 3089347.
- ^ Arutyunyan RM, Sarkisyan TF, Oganesyan GG, Durnev AD (March 1994). "Comparative investigation of anticlastogenic effects in cell cultures of healthy donors and patients with nettle-rash". Mutation Research. 320 (4): 335–41. doi:10.1016/0165-1218(94)90086-8. PMID 7508559.
- ^ Sergeeva SA, Gulyaeva IL (May 2006). "Distribution of bemitil in organs and tissues of rats after single or repeated administration". Bulletin of Experimental Biology and Medicine. 141 (5): 596–8. doi:10.1007/s10517-006-0230-0. PMID 17181062. S2CID 5826597.
- ^ "Summary of Modifications – 2018 WADA Prohibited List" (PDF). World Anti-Doping Agency. Retrieved December 1, 2020.
The following were added to evaluate misuse in sport: 2-ethylsulfanyl-1H-benzimidazole (bemitil) in- and out-of-competition
- ^ "WADA 2021 Monitoring Program" (PDF). World Anti-Doping Agency. Retrieved December 1, 2020.
The World Anti-Doping Code (Article 4.5) states: "WADA, in consultation with Signatories and governments, shall establish a monitoring program regarding substances which are not on the Prohibited List, but which WADA wishes to monitor in order to detect patterns of misuse in sport."
- ^ "World Anti-Doping Code - (WADA) Code - (USADA)". U.S. Anti-Doping Agency. Retrieved December 2, 2020.
USADA is a fully compliant signatory to the World Anti-Doping Code and WADA International Standards
