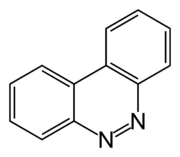
Benzo(c)cinnoline
Appearance
(Redirected from Benzocinnoline)
| |
| Names | |
|---|---|
| Preferred IUPAC name
Benzo[c]cinnoline | |
| Other names
Diphenylenazone; phenazone; 9,10-diazaphenanthrene; 2,2'-azobiphenyl; 3,4-benzocinnoline; 5,6-phenanthroline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H8N2 | |
| Molar mass | 180.21 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzo[c]cinnoline is a tricyclic organic compound with the formula C12H8N2. Formally this species is derived by oxidative dehydrogenation of 2,2'-diaminobiphenyl. This heterocycle reacts with iron carbonyls to form C12H8N2Fe2(CO)6.[1]
See also
[edit]References
[edit]- ^ R. P. Bennett, "Iron Carbonyl Complexes of Azo Compounds" Inorganic Chemistry, Volume 9, pp. 2184-6 (1970) (description of the first iron carbonyl derivative)
